
In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Tantalum is a chemical element; it has symbol Ta and atomic number 73. Previously known as tantalium, it is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48-52%) but there are also stronger solutions and pure HF has a boiling point near room temperature. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.

Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. This group is sometimes called the vanadium group or vanadium family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion. The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all commonly available elements and fluorine has the highest electronegativity.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.

Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption, which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.

Lead(II) fluoride is the inorganic compound with the formula PbF2. It is a white solid. The compound is polymorphic, at ambient temperatures it exists in orthorhombic (PbCl2 type) form, while at high temperatures it is cubic (Fluorite type).

Tantalum(V) fluoride is the inorganic compound with the formula TaF5. It is one of the principal molecular compounds of tantalum. Characteristic of some other pentafluorides, the compound is volatile but exists as an oligomer in the solid state.

Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.
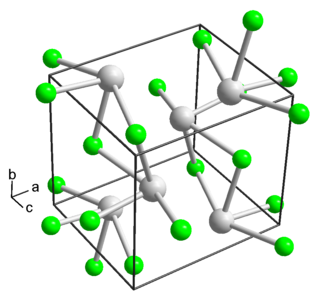
Thallium(I) fluoride is the inorganic compound with the formula TlF. It is a white solid, forming orthorhombic crystals. The solid is slightly deliquescent. It has a distorted sodium chloride (rock salt) crystal structure, due to the 6s2 inert pair on Tl+.

Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6. It is a yellow solid that is an example of a comparatively insoluble potassium salt. The salt features the hexachloroplatinate(IV) dianion, which has octahedral coordination geometry.

Potassium azide is the inorganic compound having the formula KN3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.

Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. It is a colorless solid.
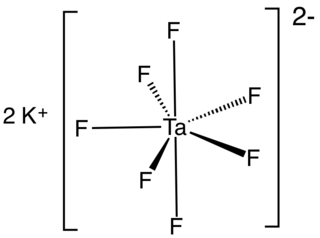
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.



















