Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
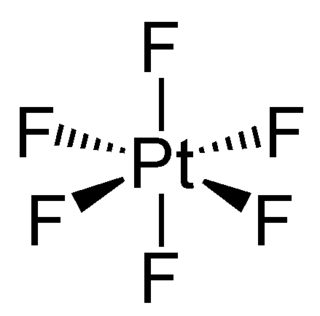
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Tantalum(V) fluoride is the inorganic compound with the formula TaF5. It is one of the principal molecular compounds of tantalum. Characteristic of some other pentafluorides, the compound is volatile but exists as an oligomer in the solid state.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This isotope of plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. It is a colourless solid and melts just below room temperature and boils in 34 °C. It is one of the seventeen known binary hexafluorides.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
Chromium hexafluoride or chromium(VI) fluoride (CrF6) is a hypothetical chemical compound between chromium and fluorine with the chemical formula CrF6. It was previously thought to be an unstable yellow solid decomposing at −100 °C, but this has been shown to be a misidentification of chromium pentafluoride, CrF5.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is a volatile orange crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.
Rhenium pentafluoride is a binary inorganic compound of rhenium and fluorine with the chemical formula ReF5. This is a salt of rhenium and hydrofluoric acid.

Molybdenum oxytetrafluoride is the inorganic compound with the formula MoOF4. It is a white, diamagnetic solid. According to X-ray crystallography, it is a coordination polymer consisting of a linear chain of alternating Mo and F atoms. Each Mo center is octahedral, the coordination sphere being defined by oxide, three terminal fluorides, and two bridging fluorides. In contrast to this motif, tungsten oxytetrafluoride crystallizes as a tetramer, again with bridging fluoride ligands.













