
Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.

Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. It is the acyl fluoride of chloric acid.

Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.

Bromine monofluoride is a quite unstable interhalogen compound with the chemical formula BrF. It can be produced through the reaction of bromine trifluoride (or bromine pentafluoride) and bromine. Due to its lability, the compound can be detected but not isolated:

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Chlorotrifluorosilane is an inorganic gaseous compound with formula SiClF3 composed of silicon, fluorine and chlorine. It is a silane that substitutes hydrogen with fluorine and chlorine atoms.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.
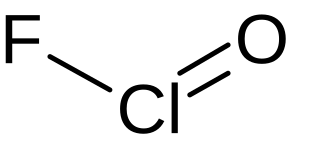
Chlorosyl fluoride is an inorganic compound of chlorine, fluorine, and oxygen with the chemical formula OClF.
Oxygen monofluoride is an unstable binary inorganic compound radical of fluorine and oxygen with the chemical formula OF. This is the simplest of many oxygen fluorides.
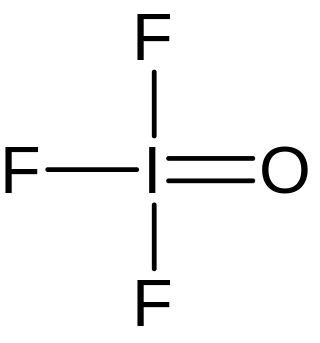
Iodosyl trifluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IOF3.
Bromosyl trifluoride is an inorganic compound of bromine, fluorine, and oxygen with the chemical formula BrOF3.
Iodine trifluoride dioxide is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F3. The compound was first obtained by Engelbrecht and Petersy in 1969.

Hexachlorodisiloxane is a chemical compound composed of chlorine, silicon, and oxygen. Structurally, it is the symmetrical ether of two trichlorosilyl groups, and can be synthesized via high-temperature oxidation of silicon tetrachloride:
Einsteinium fluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF3.











