In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
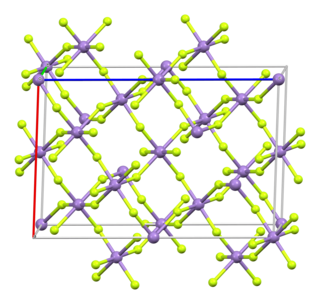
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.
Tellurium hexafluoride is the inorganic compound of tellurium and fluorine with the chemical formula TeF6. It is a colorless, highly toxic gas with an unpleasant odor.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:
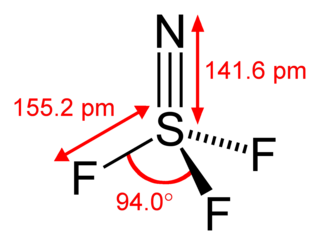
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula NSF3. It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds. It has tetrahedral molecular geometry around the sulfur atom, and is regarded to be a prime example of a compound that has a sulfur-nitrogen triple bond.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. Like other inorganic arsenic compounds, it is highly toxic.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
Palladium fluoride is the name of a series of binary compounds of palladium and fluorine. These include:

Palladium(II) fluoride, also known as palladium difluoride, is the chemical compound of palladium and fluorine with the formula PdF2.

Palladium (IV) fluoride, also known as palladium tetrafluoride, is the chemical compound of palladium and fluorine with the chemical formula PdF4. The palladium atoms in PdF4 are in the +4 oxidation state.
Nitrogen pentafluoride is a theoretical compound of nitrogen and fluorine with the chemical formula NF5. It is hypothesized to exist based on the existence of the pentafluorides of the atoms below nitrogen in the periodic table, such as phosphorus pentafluoride. Theoretical models of the nitrogen pentafluoride molecule are either a trigonal bipyramidal covalently bound molecule with symmetry group D3h, or [NF4]+F−, which would be an ionic solid.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
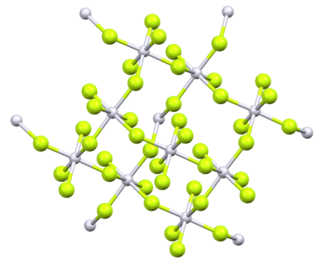
Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.
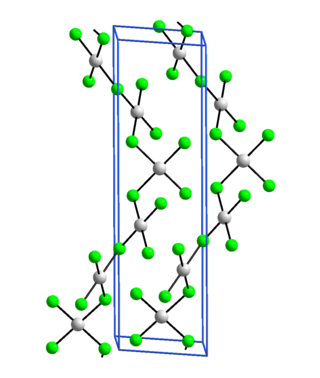
Silver(III) fluoride, AgF3, is an unstable, bright-red, diamagnetic compound containing silver in the unusual +3 oxidation state. Its crystal structure is very similar to that of gold(III) fluoride: it is a polymer consisting of rectangular AgF4 units linked into chains by fluoro bridges.
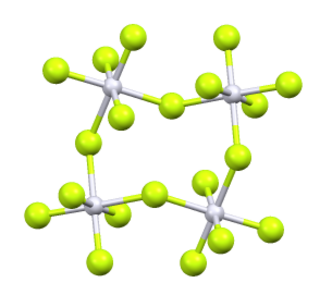
Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride.












