
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble in water. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.

Magnesium fluoride is an ionically bonded inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.

A gas laser is a laser in which an electric current is discharged through a gas to produce coherent light. The gas laser was the first continuous-light laser and the first laser to operate on the principle of converting electrical energy to a laser light output. The first gas laser, the Helium–neon laser (HeNe), was co-invented by Iranian engineer and scientist Ali Javan and American physicist William R. Bennett, Jr., in 1960. It produced a coherent light beam in the infrared region of the spectrum at 1.15 micrometres.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.

Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.

Neutron detection is the effective detection of neutrons entering a well-positioned detector. There are two key aspects to effective neutron detection: hardware and software. Detection hardware refers to the kind of neutron detector used and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.

Plutonium(IV) fluoride is a chemical compound with the formula (PuF4). This salt is generally a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants. Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.

Strontium fluoride, SrF2, also called strontium difluoride and strontium(II) fluoride, is a fluoride of strontium. It is a brittle white crystalline solid. In nature, it appears as the very rare mineral strontiofluorite.

Bismuth germanium oxide or bismuth germanate is an inorganic chemical compound of bismuth, germanium and oxygen. Most commonly the term refers to the compound with chemical formula Bi4Ge3O12 (BGO), with the cubic evlitine crystal structure, used as a scintillator. (The term may also refer to a different compound with formula Bi12GeO20, an electro-optical material with sillenite structure, and Bi2Ge3O9.)

Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relationship with aluminium.
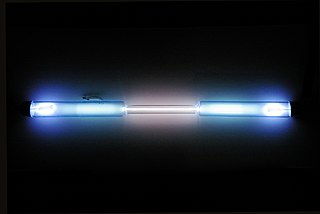
Krypton is a chemical element; it has symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert.

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.
The borate fluorides or fluoroborates are compounds containing borate or complex borate ions along with fluoride ions that form salts with cations such as metals. They are in the broader category of mixed anion compounds. They are not to be confused with tetrafluoroborates (BF4) or the fluorooxoborates which have fluorine bonded to boron.




















