Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are hygroscopic blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.
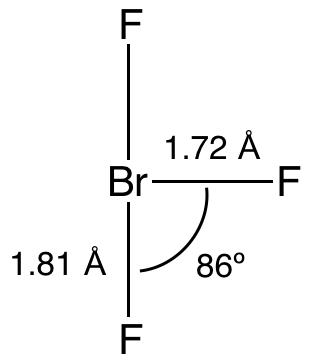
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.

Cobalt(II) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid compound which is antiferromagnetic at low temperatures (TN=37.7 K) The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate red orthogonal crystal, (CoF2·4H2O). CoF2 is used in oxygen-sensitive fields, namely metal production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can be dissolved in warm mineral acid, and will decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoF2·2H2O and tri-hydrate CoF2·3H2O forms of the compound. The hydrate will also decompose with heat.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
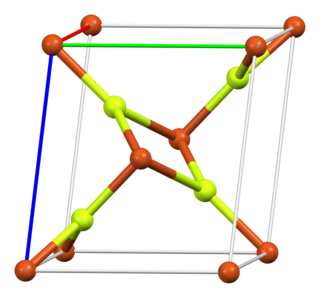
Copper(II) fluoride is an inorganic compound with the chemical formula CuF2. The anhydrous form is a white, ionic, crystalline, hygroscopic salt with a distorted rutile-type crystal structure, similar to other fluorides of chemical formulae MF2 (where M is a metal). The dihydrate, CuF2·2H2O, is blue in colour.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.
Nickel(II) iodide is an inorganic compound with the formula NiI2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above). This bluish-green colour is typical of hydrated nickel(II) compounds. Nickel iodides find some applications in homogeneous catalysis.

Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Zinc fluoride is an inorganic chemical compound with the chemical formula ZnF2. It is encountered as the anhydrous form and also as the tetrahydrate, ZnF2·4H2O (rhombohedral crystal structure). It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding. Unlike the other zinc halides, ZnCl2, ZnBr2 and ZnI2, it is not very soluble in water.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.

Rhodium(III) fluoride or rhodium trifluoride is the inorganic compound with the formula RhF3. It is a red-brown, diamagnetic solid.
Cobalt compounds are chemical compounds formed by cobalt with other elements.

















