In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
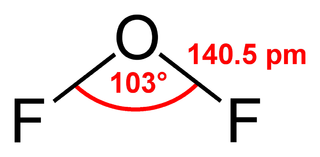
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a bent molecular geometry. It is a strong oxidizer and has attracted attention in rocketry for this reason. With a boiling point of −144.75 °C, OF2 is the most volatile (isolable) triatomic compound. The compound is one of many known oxygen fluorides.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Chromium(III) fluoride is an inorganic compound with the chemical formula CrF3. It forms several hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the hydrates [Cr(H2O)6]F3 (violet) and [Cr(H2O)6]F3·3H2O (green) are soluble in water. The anhydrous form sublimes at 1100–1200 °C.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.

Cobalt(II) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid compound which is antiferromagnetic at low temperatures (TN=37.7 K) The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate red orthogonal crystal, (CoF2·4H2O). CoF2 is used in oxygen-sensitive fields, namely metal production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can be dissolved in warm mineral acid, and will decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoF2·2H2O and tri-hydrate CoF2·3H2O forms of the compound. The hydrate will also decompose with heat.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Gallium(III) fluoride (GaF3) is a chemical compound. It is a white solid that melts under pressure above 1000 °C but sublimes around 950 °C. It has the FeF3 structure where the gallium atoms are 6-coordinate. GaF3 can be prepared by reacting F2 or HF with Ga2O3 or by thermal decomposition of (NH4)3GaF6. GaF3 is virtually insoluble in water. Solutions of GaF3 in HF can be evaporated to form the trihydrate, GaF3·3H2O, which on heating gives a hydrated form of GaF2(OH). Gallium(III) fluoride reacts with mineral acids to form hydrofluoric acid.

Actinium(III) fluoride (AcF3) is an inorganic compound, a salt of actinium and fluorine.
Praseodymium(III) fluoride is an inorganic compound with the formula PrF3, being the most stable fluoride of praseodymium.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anhydrous compound is a green powdery solid at room temperature.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion is in the -2 oxidation state. It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red, while the octahydrate is a pink solid. Both of these salts are insoluble in water.

Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.
Cobalt compounds are chemical compounds formed by cobalt with other elements.