
Promethium is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust at any given time. Promethium is one of the only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Caesium fluoride is an inorganic compound with the formula CsF. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion. The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all commonly available elements and fluorine has the highest electronegativity.
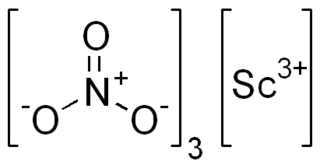
Scandium(III) nitrate, Sc(NO3)3, is an ionic compound. It is an oxidizer, as all nitrates are. The salt is applied in optical coatings, catalysts, electronic ceramics and the laser industry.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium. Several occur as minerals.

Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless salt is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.

Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It has only been obtained as an unstable pale yellow solid. The corresponding SF4 is a stable, useful reagent.
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.

Uranium trifluoride is an inorganic chemical compound with the chemical formula UF3.

Americium(III) fluoride or americium trifluoride is the chemical compound composed of americium and fluorine with the formula AmF3. It is a water soluble, pink salt.
Americium(III) iodide or americium triiodide is the chemical compound, a salt composed of americium and iodine with the formula AmI3.
Calcium monosilicide (CaSi) is an inorganic compound, a silicide of calcium. It can be prepared by reacting elemental calcium and silicon at temperatures above 1000 °C. It is a Zintl phase, where silicon has oxidation state −2 and covalence 2.
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.

Promethium(III) fluoride or promethium trifluoride is a salt of promethium and fluorine with the formula PmF3.

Curium(III) bromide is the bromide salt of curium. It has an orthorhombic crystal structure.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Cerium diiodide is an iodide of cerium, with the chemical formula of CeI2.
Promethium(III) iodide is an inorganic compound, with the chemical formula of PmI3. It is a red radioactive solid with a melting point of 695 °C.

Tungsten(II) iodide is an iodide of tungsten, with the chemical formula [W6I8]I4, or abbreviated as WI2.












