| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names Germanium tetrachloride Tetrachlorogermane Tetrachloridogermanium | |||
| Other names Germanium(IV) chloride Neutral germanium chloride (1:4) | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.093 | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| GeCl4 | |||
| Molar mass | 214.40 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 1.879 g/cm3 (20 °C) 1.844 g/cm3 (30 °C) [1] | ||
| Melting point | −49.5 °C (−57.1 °F; 223.7 K) | ||
| Boiling point | 86.5 °C (187.7 °F; 359.6 K) | ||
| Soluble, hydrolyses | |||
| Solubility | Soluble in ether, benzene, chloroform, CCl4 Very soluble in HCl, dilute H2SO4 | ||
| −72.0·10−6 cm3/mol | |||
Refractive index (nD) | 1.464 | ||
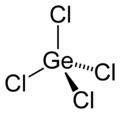
| Structure | |||
| tetrahedral [2] | |||
| Thermochemistry [3] | |||
Std molar entropy (S⦵298) | 245.6 J·mol−1·K−1 | ||
Std enthalpy of formation (ΔfH⦵298) | −531.8 kJ·mol−1 | ||
Gibbs free energy (ΔfG⦵) | −462.7 kJ·mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Reacts slowly with water to form HCl and GeO2, corrosive, lachrymator | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | "External MSDS" | ||
| Related compounds | |||
Other anions | Germanium tetrafluoride Germanium tetrabromide Germanium tetraiodide | ||
Other cations | Carbon tetrachloride Silicon tetrachloride Tin(IV) chloride Lead(IV) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Germanium tetrachloride is a colourless, fuming liquid [4] with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.