 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
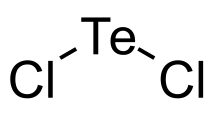
| Cl2Te | |
| Molar mass | 198.50 g·mol−1 |
| Appearance | black solid [1] |
| Density | 6.9 g·cm−3 [1] |
| Melting point | 208 °C [1] |
| Boiling point | 328 °C [1] |
| reacts [1] | |
| Solubility | reacts with diethyl ether, insoluble in tetrachloromethane [1] |
| Related compounds | |
Other anions | Ditellurium bromide, Te2Br |
Other cations | Dichlorine monoxide, OCl2 Sulfur dichloride, SCl2 Selenium dichloride, SeCl2 Polonium dichloride, PoCl2 |
Related compounds | Tritellurium dichloride, Te3Cl2 Tellurium tetrachloride, TeCl4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tellurium dichloride is a chloride of tellurium with the chemical formula TeCl2. It is a lightly characterized material that has attracted little attention.
![Structure of trans-[TeCl2(SC(NMe2)2)2] (H atoms omitted). Color code: green = Cl, dark gray = Te, yellow = S. ZZZEJK01.png](http://upload.wikimedia.org/wikipedia/commons/thumb/6/6e/ZZZEJK01.png/330px-ZZZEJK01.png)