
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin metallum ("metal") and the Greek oeides. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature.
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most [economically] important sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid.

In chemistry, tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium. It is used in making specialized glasses as well as a reagent in organic chemistry.
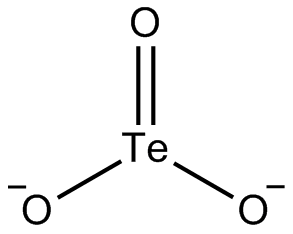
The tellurite ion is TeO2−
3. A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite. A mineral with the formula TeO2 is called tellurite.

Tellurous acid is an inorganic compound with the formula H2TeO3. It is the oxoacid of tellurium(IV). This compound is not well characterized. An alternative way of writing its formula is (HO)2TeO. In principle, tellurous acid would form by treatment of tellurium dioxide with water, that is by hydrolysis. The related conjugate base is well known in the form of several salts such as potassium hydrogen tellurite, KHTeO3.

Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium.

Tellurium tetrafluoride, TeF4, is a stable, white, hygroscopic crystalline solid and is one of two fluorides of tellurium. The other binary fluoride is tellurium hexafluoride. The widely reported Te2F10 has been shown to be F5TeOTeF5 There are other tellurium compounds that contain fluorine, but only the two mentioned contain solely tellurium and fluorine. Tellurium difluoride, TeF2, and ditellurium difluoride, Te2F2 are not known.

Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula Mn2O7. Manganese heptoxide is a volatile liquid with an oily consistency. It is a highly reactive and powerful oxidizer that reacts explosively with nearly any organic compound. It was first described in 1860. It is the acid anhydride of permanganic acid.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
Tellurium trioxide (TeO3) is an inorganic chemical compound of tellurium and oxygen. In this compound, tellurium is in the +6 oxidation state.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Tellurium compounds are compounds containing the element tellurium (Te). Tellurium belongs to the chalcogen family of elements on the periodic table, which also includes oxygen, sulfur, selenium and polonium: Tellurium and selenium compounds are similar. Tellurium exhibits the oxidation states −2, +2, +4 and +6, with +4 being most common.












