
An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Selenium is a chemical element; it has the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

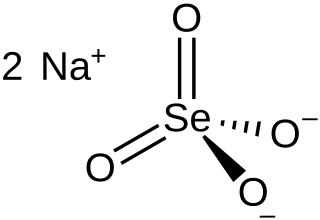
Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite. Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.

Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by O=Se(OH)2. It is the principal oxoacid of selenium; the other being selenic acid.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Hydroperoxides or peroxols are compounds of the form ROOH, where R stands for any group, typically organic, which contain the hydroperoxy functional group. Hydroperoxide also refers to the hydroperoxide anion and its salts, and the neutral hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

Selenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a colorless liquid. With a high dielectric constant (55) and high specific conductance, it is an attractive solvent. Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Sodium selenate is the inorganic compound with the formula Na
2SeO
4. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.

Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilicity is often stated to be related to the hardness of the element, within the HSAB theory, but it has been shown that oxophilicity depends more on the electronegativity and effective nuclear charge of the element than on its hardness. This explains why the early transition metals, whose electronegativities and effective nuclear charges are low, are very oxophilic. Many main group compounds are also oxophilic, such as derivatives of aluminium, silicon, and phosphorus(III). The handling of oxophilic compounds often requires air-free techniques.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.

Potassium selenate, K
2SeO
4, is an odorless, white solid that forms as the potassium salt of selenic acid.
The Riley oxidation is a selenium dioxide-mediated oxidation of methylene groups adjacent to carbonyls. It was first reported by Harry Lister Riley and co-workers in 1932. In the decade that ensued, selenium-mediated oxidation rapidly expanded in use, and in 1939, Andre Guillemonat and co-workers disclosed the selenium dioxide-mediated oxidation of olefins at the allylic position. Today, selenium-dioxide-mediated oxidation of methylene groups to alpha ketones and at the allylic position of olefins is known as the Riley Oxidation.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.





















