 WSe2 monolayer on graphene (yellow) and its atomic image (inset) [1] | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.877 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| WSe2 | |
| Molar mass | 341.76 g/mol |
| Appearance | grey to black solid |
| Odor | odorless |
| Density | 9.32 g/cm3 [2] |
| Melting point | > 1200 °C |
| insoluble | |
| Band gap | ~1 eV (indirect, bulk) [3] ~1.7 eV (direct, monolayer) [4] |
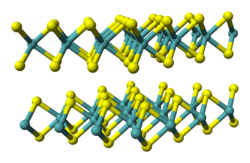
| Structure | |
| hP6, space group P6 3/mmc, No 194 [2] | |
a = 0.3297 nm, c = 1.2982 nm | |
| Trigonal prismatic (WIV) Pyramidal (Se2−) | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) | −185.3 kJ mol−1 [5] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | External MSDS |
| GHS labelling: | |
   | |
| Warning | |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+P316, P304+P340, P316, P319, P321, P330, P391, P403+P233, P405, P501 | |
| Related compounds | |
Other anions | Tantalum diselenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tungsten diselenide is an inorganic compound with the formula WSe2. [6] The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. [7] It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.
