
Perrhenic acid is the chemical compound with the formula Re2O7(H2O)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4·H2O are formed, which contain tetrahedral ReO−4. For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

In chemistry, tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.
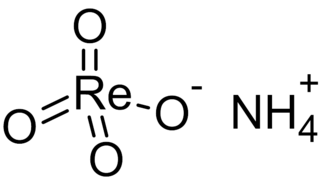
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
Lithium carbide, Li2C2, often known as dilithium acetylide, is a chemical compound of lithium and carbon, an acetylide. It is an intermediate compound produced during radiocarbon dating procedures. Li2C2 is one of an extensive range of lithium-carbon compounds which include the lithium-rich Li4C, Li6C2, Li8C3, Li6C3, Li4C3, Li4C5, and the graphite intercalation compounds LiC6, LiC12, and LiC18.
Calcium selenide (CaSe) is a chemical compound consisting of the elements calcium and selenium in equal stoichiometric ratio.

Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.

Rubidium ozonide is an oxygen rich compound of rubidium. It is an ozonide, meaning it contains the ozonide anion (O3−).

Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid.

Sulfur dicyanide is an inorganic compound with the formula S(CN)2. A white, slightly unstable solid, the compound is mainly of theoretical and fundamental interest given its simplicity. It is the first member of the dicyanosulfanes Sx(CN)2, which includes thiocyanogen ((SCN)2) and higher polysulfanes up to S4(CN)2. According to X-ray crystallography, the molecule is planar, the SCN units are linear, with an S-C-S angle of 95.6°.
Beryllium chromate is a hypothetical inorganic compound, with the chemical formula of BeCrO4. It is predicted to have a certain bonding ability with noble gases. Little evidence has been published supporting the existence of this material.

Rubidium selenide is an inorganic compound composed of selenium and rubidium. It is a selenide with a chemical formula of Rb2Se. Rubidium selenide is used together with caesium selenide in photovoltaic cells.

Strontium selenide is an inorganic compound with the chemical formula SrSe.










