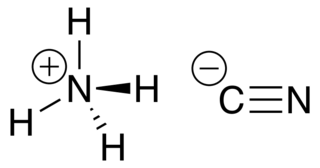Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has the third-highest melting point and second-highest boiling point of any element at 5869 K. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
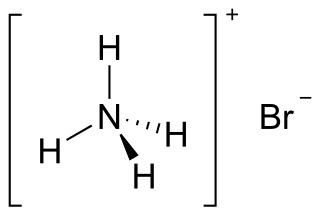
Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of traces of bromide (Br−) to bromine (Br2).

Ammonium chlorate is an inorganic compound with the formula NH4ClO3.

Perrhenic acid is the chemical compound with the formula Re2O7(H2O)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4·H2O are formed, which contain tetrahedral ReO−4. For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.

Tetra ammine copper(II) sulphate is the salt with the formula [Cu(NH3)4]SO4·H2O. This dark blue to purple solid is a salt of the metal complex [Cu(NH3)4(H2O)]2+. It is closely related to Schweizer's reagent, which is used for the production of cellulose fibers in the production of rayon.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2[ReH9]. This colourless salt is soluble in water but only poorly soluble in most alcohols. This salt contains the nonahydridorhenate(VII) anion, [ReH9]2−, which is a rare example of a coordination complex bearing only hydride ligands.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.

Oxotrichlorobis(triphenylphosphine)rhenium(V) is the chemical compound with the formula ReOCl3(PPh3)2. This yellow, air-stable solid is a precursor to a variety of other rhenium complexes. In this diamagnetic compound, Re has an octahedral coordination environment with one oxo, three chloro and two mutually trans triphenylphosphine ligands. The oxidation state of rhenium is +5 and its configuration is d2.
The perrhenate ion is the anion with the formula ReO−
4, or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable over a broad pH range and can be precipitated from solutions with the use of organic cations. At normal pH, perrhenate exists as metaperrhenate, but at high pH mesoperrhenate forms. Perrhenate, like its conjugate acid perrhenic acid, features rhenium in the oxidation state of +7 with a d0 configuration. Solid perrhenate salts takes on the color of the cation.
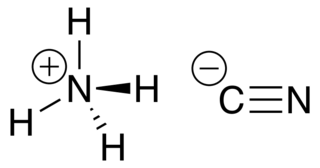
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.
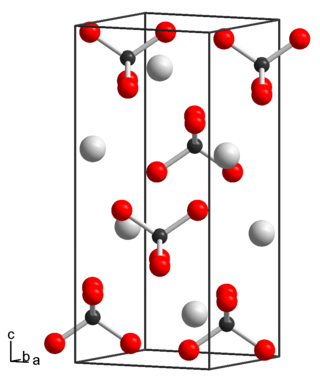
Potassium perrhenate is an inorganic compound with the chemical formula KReO4.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.
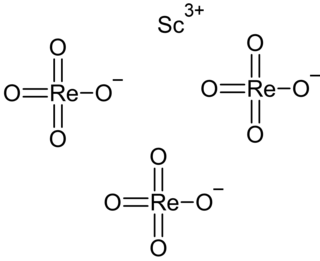
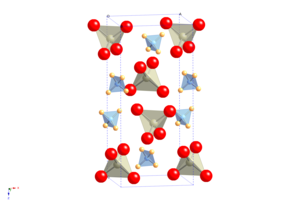
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3. Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.
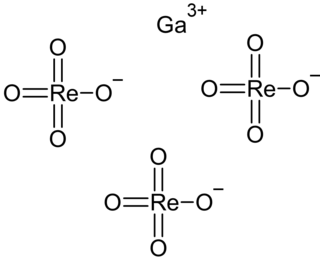
Gallium perrhenate is an inorganic compound with the chemical formula of Ga(ReO4)3. It exists in the anhydrous and hydrate forms.

Rhenium(III) iodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI
3.