 | |
 | |
| Names | |
|---|---|
| IUPAC name Ammonium hexachloroplatinate(IV) | |
| Other names ammonium chloroplatinate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.233 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
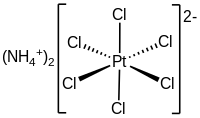
| (NH4)2PtCl6 | |
| Molar mass | 443.87 g/mol |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 3.065 g/cm3 |
| Melting point | 380 °C (716 °F; 653 K) decomposes |
| 0.289 g/100ml (0 °C) 0.7 g/100ml (15 °C) [1] 0.499 g/100ml (20 °C) 3.36 g/100ml (100 °C) | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H290, H301, H317, H318, H334 | |
| P234, P261, P264, P270, P272, P280, P285, P301+P310, P302+P352, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 195 mg/kg rat |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2[PtCl6]. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.