
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.

Rhodium is a chemical element with the symbol Rh and atomic number 45. It is an extraordinarily rare, silvery-white, hard, corrosion-resistant, and chemically inert transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, which is 103Rh. Naturally occurring rhodium is usually found as a free metal, as an alloy with similar metals, and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.
Benzonitrile is the chemical compound with the formula C
6H
5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.

Tungsten(V) bromide is the inorganic compound with the empirical formula WBr5. The compound consists of bioctahedral structure, with two bridging bromide ligands, so its molecular formula is W2Br10.

Iron(II) bromide is an inorganic compound with the chemical formula FeBr2. The anhydrous compound is a yellow or brownish-colored paramagnetic solid. Several hydrates of FeBr2 are also known, all being pale colored solids. It is a common precursor to other iron compounds in research laboratory, but no applications exist for this compound.
The Buchwald–Hartwig amination is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods for the synthesis of aromatic C–N bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods while significantly expanding the repertoire of possible C–N bond formation.

Palladium(II) bis(acetylacetonate) is a compound with formula Pd(C5H7O2)2. This yellow solid is the most common palladium complex of acetylacetonate. This compound is commercially available and used as a catalyst precursor in organic synthesis. The molecule is relatively planar with idealized D2h symmetry.
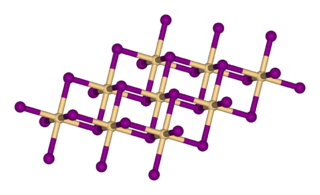
Manganese(II) bromide is the chemical compound composed of manganese and bromine with the formula MnBr2.

Platinum(II) acetate is a purple-colored coordination complex. The complex adopts an unusual structure consisting of a square array of Pt atoms.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.

Sodium tetrachloropalladate is an inorganic compound with the chemical formula Na2PdCl4. This salt, and the analogous alkali metal salts of the form M2PdCl4, may be prepared simply by reacting palladium(II) chloride with the appropriate alkali metal chloride in aqueous solution. Palladium(II) chloride is insoluble in water, whereas the product dissolves:
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Palladium(II) bromide is an inorganic compound of palladium and bromine with the chemical formula PdBr2. It is a commercially available, though less common than palladium(II) chloride, the usual entry point to palladium chemistry. Unlike the chloride, palladium(II) bromide is insoluble in water, but dissolves when heated in acetonitrile to give monomeric acetonitrile adducts:

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. Covalently bonded metal halides may be discrete molecules, such as uranium hexafluoride, or they may form polymeric structures, such as palladium chloride.

Platinum(II) bis(acetylacetonate) is the coordination compound with the formula Pt(O2C5H7)2, abbreviated Pt(acac)2. The homoleptic acetylacetonate complex of platinum(II), it is a yellow, benzene-soluble solid. According to X-ray crystallography, the Pt center is square planar. The compound is a widely used precursor to platinum-based catalysts.
Rhodium(IV) fluoride is a chemical compound of rhodium and fluorine. It is formed when rhodium(III) bromide reacts with bromine trifluoride. Iridium(IV) fluoride, palladium(IV) fluoride and platinum(IV) fluoride have the same crystal structure.
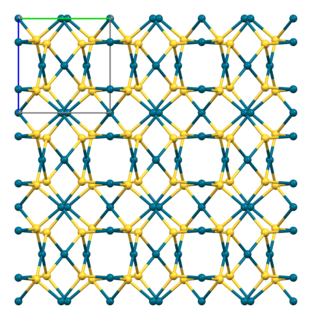
Palladium(II) sulfide is a chemical compound of palladium and sulfur with the chemical formula PdS. Like other palladium and platinum chalcogenides, palladium(II) sulfide has complex structural, electrical and magnetic properties.














