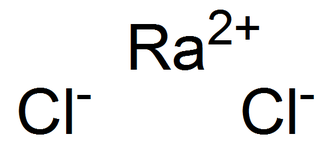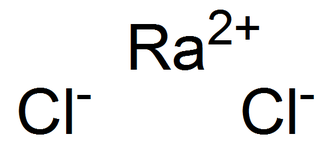
Radium chloride is an inorganic compound with the chemical formula RaCl2. It is a radium salt of hydrogen chloride. It was the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.

Barium carbonate is the inorganic compound with the formula BaCO3. Like most alkaline earth metal carbonates, it is a white salt that is poorly soluble in water. It occurs as the mineral known as witherite. In a commercial sense, it is one of the most important barium compounds.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −2.7) and an oxidizing agent.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Strontium chloride (SrCl2) is a salt of strontium and chloride. It is a 'typical' salt, forming neutral aqueous solutions. As with all compounds of strontium, this salt emits a bright red colour in flame, and is commonly used in fireworks to that effect. Its properties are intermediate between those for barium chloride, which is more toxic, and calcium chloride.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane and alcohols. The formula for this acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2, which highlights its monoprotic character. Salts derived from this acid are called hypophosphites.

Zinc bromide (ZnBr2) is an inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride (ZnCl2), namely a high solubility in water forming acidic solutions, and good solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr2·2H2O.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Radium bromide is the bromide salt of radium, with the formula RaBr2. It is produced during the process of separating radium from uranium ore. This inorganic compound was discovered by Pierre and Marie Curie in 1898, and the discovery sparked a huge interest in radiochemistry and radiotherapy. Since elemental radium oxidizes readily in air and water, radium salts are the preferred chemical form of radium to work with. Even though it is more stable than elemental radium, radium bromide is still extremely toxic, and can explode under certain conditions.

Lead(II) bromide is the inorganic compound with the formula PbBr2. It is a white powder. It is produced in the burning of typical leaded gasolines.

Dithionic acid, H2S2O6, is the inorganic compound with the formula H2S2O6. It is the doubly protonated derivative of dithionate, a well-characterized dianion. Dithionic acid is mainly observed and characterized as an aqueous solution.

Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3.

Rhodium(III) bromide refers to inorganic compounds of the formula RhBr3(H2O)n where n = 0 or approximately three. Both forms are brown solids. The hydrate is soluble in water and lower alcohols. It is used to prepare rhodium bromide complexes. Rhodium bromides are similar to the chlorides, but have attracted little academic or commercial attention.
Radium compounds are compounds containing the element radium (Ra). Due to radium's radioactivity, not many compounds have been well characterized. Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-radiolysis from radium's alpha decay. Insoluble radium compounds coprecipitate with all barium, most strontium, and most lead compounds.