 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.211 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 1746 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| BrF3 | |
| Molar mass | 136.90 g/mol |
| Appearance | straw-coloured liquid hygroscopic |
| Odor | Choking, pungent [1] |
| Density | 2.803 g/cm3 [2] |
| Melting point | 8.77 °C (47.79 °F; 281.92 K) |
| Boiling point | 125.72 °C (258.30 °F; 398.87 K) |
| Reacts with water [3] | |
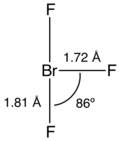
| Structure | |
| T-shaped (C2v) | |
| 1.19 D | |
| Hazards [4] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Reacts violently with water to release HF, highly toxic, corrosive, powerful oxidizer |
| GHS labelling: | |
    | |
| Danger | |
| H271, H300+H310+H330, H314, H373 | |
| P102, P103, P210, P220, P221, P260, P264, P271, P280, P283, P284, P301+P310, P301+P330+P331, P303+P361+P353, P304+P312, P305+P351+P338+P310, P306+P360, P308+P313, P340, P363, P370+P380 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | http://www.chammascutters.com/en/downloads/Bromine-Trifluoride-MSDS.pdf |
| Related compounds | |
Other anions | Bromine monochloride |
Other cations | Chlorine trifluoride Iodine trifluoride |
Related compounds | Bromine monofluoride Bromine pentafluoride |
| Supplementary data page | |
| Bromine trifluoride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor [5] which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel. [6]
