
The noble gases are the members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some cases, oganesson (Og). Under standard conditions, the first six of these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points. The properties of the seventh, unstable, element, Og, are uncertain.
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only 222Rn has a sufficiently long half-life for it to be released from the soil and rock where it is generated. Radon isotopes are the immediate decay products of radium isotopes. The instability of 222Rn, its most stable isotope, makes radon one of the rarest elements. Radon will be present on Earth for several billion more years despite its short half-life, because it is constantly being produced as a step in the decay chains of 238U and 232Th, both of which are abundant radioactive nuclides with half-lives of at least several billion years. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead. 222Rn occurs in significant quantities as a step in the normal radioactive decay chain of 238U, also known as the uranium series, which slowly decays into a variety of radioactive nuclides and eventually decays into stable 206Pb. 220Rn occurs in minute quantities as an intermediate step in the decay chain of 232Th, also known as the thorium series, which eventually decays into stable 208Pb.

Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.
Oganesson is a synthetic chemical element; it has symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scientists. In December 2015, it was recognized as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP. It was formally named on 28 November 2016. The name honors the nuclear physicist Yuri Oganessian, who played a leading role in the discovery of the heaviest elements in the periodic table.
Copernicium is a synthetic chemical element; it has symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of approximately 30 seconds. Copernicium was first created in February 1996 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It was named after the astronomer Nicolaus Copernicus on his 537th anniversary.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 8 or 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).
In chemistry, a hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride, sulfur hexafluoride, chlorine trifluoride, the chlorite ion in chlorous acid and the triiodide ion are examples of hypervalent molecules.

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
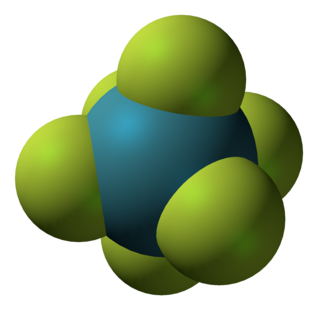
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also known as the Pimentel–Rundle three-center model after the work published by George C. Pimentel in 1951, which built on concepts developed earlier by Robert E. Rundle for electron-deficient bonding. An extended version of this model is used to describe the whole class of hypervalent molecules such as phosphorus pentafluoride and sulfur hexafluoride as well as multi-center π-bonding such as ozone and sulfur trioxide.
Quantum chemistry composite methods are computational chemistry methods that aim for high accuracy by combining the results of several calculations. They combine methods with a high level of theory and a small basis set with methods that employ lower levels of theory with larger basis sets. They are commonly used to calculate thermodynamic quantities such as enthalpies of formation, atomization energies, ionization energies and electron affinities. They aim for chemical accuracy which is usually defined as within 1 kcal/mol of the experimental value. The first systematic model chemistry of this type with broad applicability was called Gaussian-1 (G1) introduced by John Pople. This was quickly replaced by the Gaussian-2 (G2) which has been used extensively. The Gaussian-3 (G3) was introduced later.

A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple bonds are the diatomic dimolybdenum (Mo2) and ditungsten (W2), which exist in the gaseous phase and have boiling points of 4,639 °C (8,382 °F) and 5,930 °C (10,710 °F) respectively.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Iridium hexafluoride, also iridium(VI) fluoride, (IrF6) is a compound of iridium and fluorine and one of the seventeen known binary hexafluorides. It is one of only a few compounds with iridium in the oxidation state +6.

Nitrosonium octafluoroxenate(VI) is a chemical compound of xenon with nitrogen, oxygen, and fluorine, having formula (NO)
2XeF
8. It is an ionic compound containing well-separated nitrosonium cations (NO+) and octafluoroxenate(VI) anions (XeF2−
8). The molecular geometry of the octafluoroxenate(VI) ion is square antiprismatic, having Xe–F bond lengths of 1.971 Å, 1.946 Å, 1.958 Å, 2.052 Å, and 2.099 Å.

Veli Pekka Pyykkö is a Finnish academic. He was professor of Chemistry at the University of Helsinki. From 2009–2012, he was the chairman of the International Academy of Quantum Molecular Science. He is known for his extension to the periodic table of elements, known as the Pyykkö model.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Radon compounds are chemical compounds formed by the element radon (Rn). Radon is a noble gas, i.e. a zero-valence element, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas under normal circumstances, and its decay-chain parents are not, it can readily be extracted from them for research.











