In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

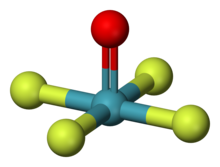
Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
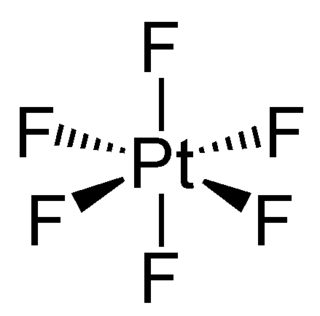
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.

Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.
Xenon compounds are compounds containing the element xenon (Xe). After Neil Bartlett's discovery in 1962 that xenon can form chemical compounds, a large number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative atoms fluorine or oxygen. The chemistry of xenon in each oxidation state is analogous to that of the neighboring element iodine in the immediately lower oxidation state.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide. It has the structure F−N=N−F and exists in both cis and trans isomers, as typical for diimides.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
Organoxenon chemistry is the study of the properties of organoxenon compounds, which contain carbon to xenon chemical bonds. The first organoxenon compounds were divalent, such as (C6F5)2Xe. The first tetravalent organoxenon compound, [C6F5XeF2][BF4], was synthesized in 2004. So far, more than one hundred organoxenon compounds have been researched.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.
Xenon nitrate, also called xenon dinitrate, is an inorganic compound consisting of one xenon atom bonded to two nitrate groups. It can be made by reacting xenon difluoride with anhydrous nitric acid, but it only exists transiently before decomposing, and therefore it has not been isolated and fully characterized. A related compound, xenon fluoride nitrate, has been made and is stable enough to be studied in more detail.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.

Xenon oxydifluoride is an inorganic compound with the molecular formula XeOF2. The first definitive isolation of the compound was published on 3 March 2007, producing it by the previously-examined route of partial hydrolysis of xenon tetrafluoride.

Seleninyl fluoride is an oxyfluoride of selenium with the chemical formula SeOF2.

Molybdenum oxytetrafluoride is the inorganic compound with the formula MoOF4. It is a white, diamagnetic solid. According to X-ray crystallography, it is a coordination polymer consisting of a linear chain of alternating Mo and F atoms. Each Mo center is octahedral, the coordination sphere being defined by oxide, three terminal fluorides, and two bridging fluorides. In contrast to this motif, tungsten oxytetrafluoride crystallizes as a tetramer, again with bridging fluoride ligands.