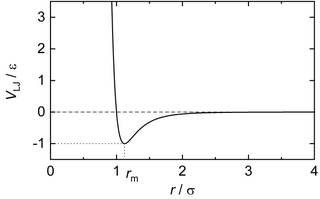
In computational chemistry, molecular physics, and physical chemistry, the Lennard-Jones potential is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is probably the one that has been the most extensively studied. It is considered an archetype model for simple yet realistic intermolecular interactions. The Lennard-Jones potential is often used as a building block in molecular models for more complex substances. Many studies of the idealized "Lennard-Jones substance" use the potential to understand the physical nature of matter.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

In chemistry, triiodide usually refers to the triiodide ion, I−
3. This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+[I3]−) and ammonium triiodide ([NH4]+[I3]−). Triiodide is observed to be a red colour in solution.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Xenon oxytetrafluoride is an inorganic chemical compound. It is an unstable colorless liquid with a melting point of −46.2 °C that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica or NaNO
3:

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.
Krypton is a chemical element; it has symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert.

Giacinto Scoles was an Italian-American chemist and physicist who was best known for his pioneering development of molecular beam methods for the study of weak van der Waals forces between atoms, molecules, and surfaces. He developed the cryogenic bolometer as a universal detector of atomic and molecule beams that not only can detect a small flux of molecules, but also responds to the internal energy of the molecules. This is the basis for the optothermal spectroscopy technique which Scoles and others have used to obtain very high signal-to noise and high resolution ro-vibrational spectra.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This isotope of plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
Cerium(III) bromide is an inorganic compound with the formula CeBr3. This white hygroscopic solid is of interest as a component of scintillation counters.
BigDFT is a free software package for physicists and chemists, distributed under the GNU General Public License, whose main program allows the total energy, charge density, and electronic structure of systems made of electrons and nuclei to be calculated within density functional theory (DFT), using pseudopotentials, and a wavelet basis.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
Thorium monoxide, is the binary oxide of thorium having chemical formula ThO. The covalent bond in this diatomic molecule is highly polar. The effective electric field between the two atoms has been calculated to be about 80 gigavolts per centimeter, one of the largest known internal effective electric fields.
Erin Johnson is a Canadian computational chemist. She holds the Herzberg–Becke Chair at Dalhousie University. She works on density functional theory and intermolecular interactions.
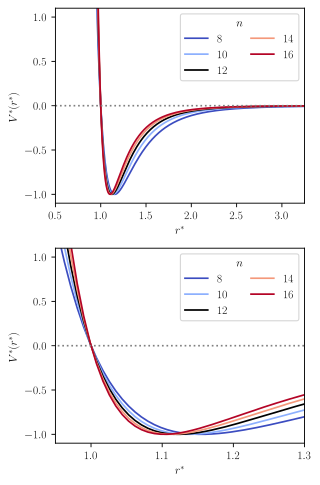
The Mie potential is an interaction potential describing the interactions between particles on the atomic level. It is mostly used for describing intermolecular interactions, but at times also for modeling intramolecular interaction, i.e. bonds.

John Lafayette Magee was an American chemist known for his work on kinetic models of radiation chemistry, especially the Samuel-Magee model for describing radiolysis in solution.
Vibrational spectroscopic maps are a series of ab initio, semiempirical, or empirical models tailored to specific IR probes to describe vibrational solvatochromic effects on molecular spectra quantitatively.












