
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is an extraordinarily rare, silvery-white, hard, corrosion-resistant, and chemically inert transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, which is 103Rh. Naturally occurring rhodium is usually found as a free metal, as an alloy with similar metals, and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Plutonium(IV) fluoride is a chemical compound with the formula (PuF4). It is a brown solid but can appear a variety of colors depending on the grain size, purity, moisture content, lighting, and presence of contaminants. Its primary use in the United States has been as an intermediary product in the production of plutonium metal for nuclear weapons usage.

Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2O3. It is a gray solid that is insoluble in ordinary solvents.
Vanadium(IV) fluoride (VF4) is an inorganic compound of vanadium and fluorine. It is paramagnetic yellow-brown solid that is very hygroscopic. Unlike the corresponding vanadium tetrachloride, the tetrafluoride is not volatile because it adopts a polymeric structure. It decomposes before melting.

Indium(III) fluoride or indium trifluoride is the inorganic compound with the formula InF3. It is a white solid.

Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.
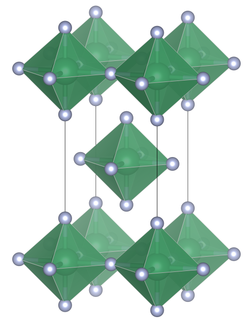
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4 and is a white solid with a melting point above 700 °C.

Palladium(II) fluoride, also known as palladium difluoride, is the chemical compound of palladium and fluorine with the formula PdF2.

Palladium(II,IV) fluoride, also known as palladium trifluoride, is a chemical compound of palladium and fluorine. It has the empirical formula PdF3, but is better described as the mixed-valence compound palladium(II) hexafluoropalladate(IV), PdII[PdIVF6], and is often written as Pd[PdF6] or Pd2F6.

Palladium(IV) fluoride, also known as palladium tetrafluoride, is the chemical compound of palladium and fluorine with the chemical formula PdF4. The palladium atoms in PdF4 are in the +4 oxidation state.

Rhodium hexafluoride, also rhodium(VI) fluoride, (RhF6) is the inorganic compound of rhodium and fluorine. A black volatile solid, it is a highly reactive material, and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluoride.

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.

Lead tetrafluoride is a compound of lead and fluorine. The yellow solid is the only room-temperature stable tetrahalide of lead. Lead tetrafluoride is isostructural with tin(IV) fluoride and contains planar layers of octahedrally coordinated lead, where the octahedra share four corners and there are two terminal, unshared, fluorine atoms trans to one another.

Chromium(IV) fluoride is an inorganic compound with the chemical formula CrF4. It has a dark greenish-black color when solid. It rapidly hydrolysizes in presence of moisture in air or directly in water.

Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.

Neptunium(IV) fluoride or neptunium tetrafluoride is a inorganic compound with the formula NpF4. It is a green salt and is isostructural with UF4.

Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.

Rhodium(III) bromide refers to inorganic compounds of the formula RhBr3(H2O)n where n = 0 or approximately three. Both forms are brown solids. The hydrate is soluble in water and lower alcohols. It is used to prepare rhodium bromide complexes. Rhodium bromides are similar to the chlorides, but have attracted little academic or commercial attention.

Praseodymium(IV) fluoride (also praseodymium tetrafluoride) is a binary inorganic compound, a highly oxidised metal salt of praseodymium and fluoride with the chemical formula PrF4. The compound forms light yellow crystals.















