
Bismuth subsalicylate, sold as generic and under the brand name Pepto-Bismol, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as diarrhea, indigestion, heartburn and nausea. It is also commonly known as pink bismuth.

Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Bismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

Bismuth telluride (Bi2Te3) is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. Bi2Te3 is a topological insulator, and thus exhibits thickness-dependent physical properties.

Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula BiH3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 °C. This compound adopts the expected pyramidal structure with H-Bi-H angles of around 90°.
Bismuth-209 (209Bi) is the isotope of bismuth with the longest known half-life of any radioisotope that undergoes α-decay. It has 83 protons and a magic number of 126 neutrons, and an atomic mass of 208.9803987 amu. Primordial bismuth consists entirely of this isotope.
Bismuth ferrite (BiFeO3, also commonly referred to as BFO in materials science) is an inorganic chemical compound with perovskite structure and one of the most promising multiferroic materials. The room-temperature phase of BiFeO3 is classed as rhombohedral belonging to the space group R3c. It is synthesized in bulk and thin film form and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K ) and ferroelectric Curie temperature are well above room temperature (approximately 1100K). Ferroelectric polarization occurs along the pseudocubic direction ( ) with a magnitude of 90–95 μC/cm2.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.
Bismuth subcarbonate (BiO)2CO3, sometimes written Bi2O2(CO3) is a chemical compound of bismuth containing both oxide and carbonate anions. Bismuth is in the +3 oxidation state. Bismuth subcarbonate occurs naturally as the mineral bismutite. Its structure consists of Bi-O layers and CO3 layers and is related to kettnerite, CaBi(CO3)OF. It is light-sensitive.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649°C.

Bismuth(III) sulfide is a chemical compound of bismuth and sulfur. It occurs in nature as the mineral bismuthinite.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black solid is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.

Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a pentavalent post-transition metal and one of the pnictogens with chemical properties resembling its lighter homologs arsenic and antimony. Elemental bismuth may occur naturally, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery white color when freshly produced, but surface oxidation can give it a pink tinge. Bismuth is the most naturally diamagnetic element, and has one of the lowest values of thermal conductivity among metals.
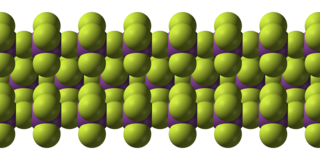
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.
Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. According to one reviewer, applications are rare even though bismuth and bismuth compounds are the least toxic among the heavy metals and are relatively cheap. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is very limited. Organobismuth heterocycles are bismole and bismabenzene. For reviews, see the cited articles

Bismuth oxychloride is an inorganic compound of bismuth with the formula BiOCl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. It is also known as pearl white.

Bismuth tribromide is an inorganic chemical compound of bismuth and bromine with the chemical formula BiBr3. It may be formed by the reaction of bismuth oxide and hydrobromic acid with the equation
Bismuth selenide (Bi2Se3) is a gray compound of bismuth and selenium also known as bismuth(III) selenide. It is a semiconductor and a thermoelectric material. While perfect stoichiometric bismuth selenide should be a semiconductor (with a gap of 0.3 eV) naturally occurring selenium vacancies act as electron donors and it often acts as a semimetal. Topologically protected surface states have been observed in Bismuth selenide, which is the subject of ongoing scientific research.
Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use.

Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.

Bismuth titanate or bismuth titanium oxide is a solid inorganic compound of bismuth, titanium and oxygen with the chemical formula of Bi12TiO20, Bi 4Ti3O12 or Bi2Ti2O7













