 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl- 1κ2C,2κ2C,3κ2C,4κ3C-[Td-(13)-Δ4-closo]- | |
| Other names rhodium(0) carbonyl; rhodium carbonyl; rhodium dodecacarbonyl | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.039.232 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
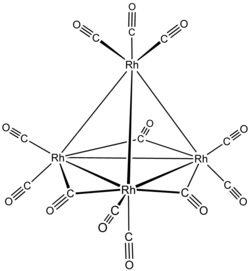
| Rh4(CO)12 | |
| Molar mass | 747.743 g/mol |
| Appearance | Red crystals |
| Solubility | Chlorocarbons, toluene, tetrahydrofuran |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
| Related compounds | |
Other cations | Tetracobalt dodecacarbonyl, Tetrairidium dodecacarbonyl |
Related compounds | Rhodium(III) chloride, Rh6(CO)16, Rh2(CO)4Cl2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic synthesis.