
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 kg/m3 (22 lb/cu yd). It is the densest known gas under standard ambient temperature and pressure. WF6 is commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
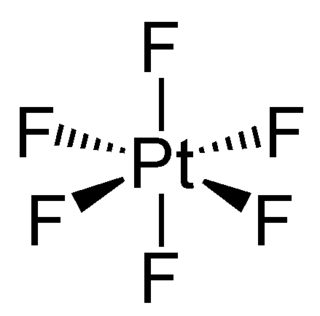
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.
Rhenium pentachloride is an inorganic compound with the formula Re2Cl10. This red-brown solid is paramagnetic.

Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron diffraction at 1.5 K. The structure is non-rigid, as evidenced by electron diffraction studies.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Iridium hexafluoride, also iridium(VI) fluoride, (IrF6) is a compound of iridium and fluorine and one of the seventeen known binary hexafluorides. It is one of only a few compounds with iridium in the oxidation state +6.

Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. It is a colourless solid and melts just below room temperature and boils in 34 °C. It is one of the seventeen known binary hexafluorides.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.

Rhodium hexafluoride, also rhodium(VI) fluoride, (RhF6) is the inorganic compound of rhodium and fluorine. A black volatile solid, it is a highly reactive material which starts to slowly thermally decompose already at room temperature and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluorides.

Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides.
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.

Osmium(V) chloride is an inorganic chemical compound of osmium metal and chlorine with the chemical formula OsCl5.
Osmium heptafluoride is a possible inorganic chemical compound of osmium metal and fluorine with the chemical formula OsF
7. It was first reported in 1966 by the reaction of fluorine and osmium at 600 °C and 400 atm, but no purported synthesis could be reproduced in 2006, giving only osmium hexafluoride instead.
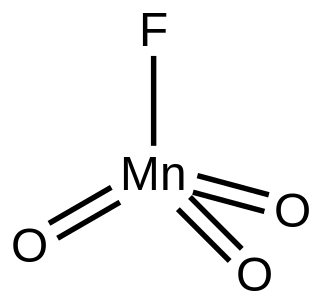
Manganese trioxide fluoride is an inorganic compound with the formula MnO3F. A green diamagnetic liquid, the compound has no applications, but it is of some academic interest as a rare example of a metal trioxide fluoride.













