
In coordination chemistry, the term scorpionate ligand refers to a tridentate (three-donor-site) ligand which would bind to a metal in a fac manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then little-known DuPont chemist of Ukrainian descent, Swiatoslaw Trofimenko. Trofimenko called this discovery "a new and fertile field of remarkable scope".

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.

Triosmium dodecacarbonyl is a chemical compound with the formula Os3(CO)12. This yellow-colored metal carbonyl cluster is an important precursor to organo-osmium compounds. Many of the advances in cluster chemistry have arisen from studies on derivatives of Os3(CO)12 and its lighter analogue Ru3(CO)12.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Beryllium hydride is an inorganic compound with the chemical formula n. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60.
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. Carbido complexes are a molecular subclass of carbides, which are prevalent. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis and related catalytic processes. They are also used as precursors for the synthesis of more complicated carbides. They are analogous to metal nitrido complexes.

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

Phosphenium ions, not to be confused with phosphonium or phosphirenium, are divalent cations of phosphorus of the form [PR2]+. Phosphenium ions have long been proposed as reaction intermediates.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
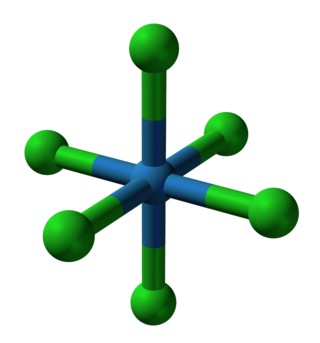
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.
Phosphanides are chemicals containing the [PH2]− anion. This is also known as the phosphino anion or phosphido ligand. The IUPAC name can also be dihydridophosphate(1−).

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.















