
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.
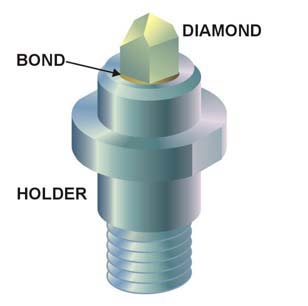
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Rhenium diboride (ReB2) is a synthetic superhard material. It was first synthesized in 1962 and re-emerged recently due to hopes of achieving high hardness comparable to that of diamond. The reported ultrahigh hardness has been questioned, although this is a matter of definition as in the initial test rhenium diboride was able to scratch diamond.

Aluminium diboride (AlB2) is a chemical compound made from the metal aluminium and the metalloid boron. It is one of two compounds of aluminium and boron, the other being AlB12, which are both commonly referred to as aluminium boride.
Aluminium dodecaboride is a superhard chemical compound with 17% aluminium content by weight.
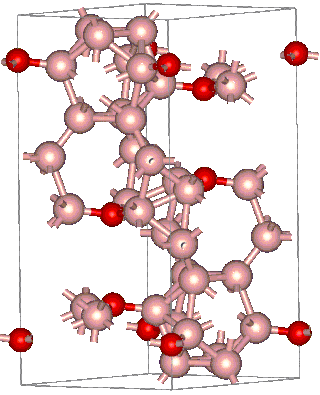
Boron suboxide (chemical formula B6O) is a solid compound with a structure built of eight icosahedra at the apexes of the rhombohedral unit cell. Each icosahedron is composed of twelve boron atoms. Two oxygen atoms are located in the interstices along the [111] rhombohedral direction. Due to its short interatomic bond lengths and strongly covalent character, B6O displays a range of outstanding physical and chemical properties such as great hardness (close to that of rhenium diboride and boron nitride), low mass density, high thermal conductivity, high chemical inertness, and excellent wear resistance.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.
Aluminium magnesium boride or Al3Mg3B56, colloqually known as BAM, is a chemical compound of aluminium, magnesium and boron. Whereas its nominal formula is AlMgB14, the chemical composition is closer to Al0.75Mg0.75B14. It is a ceramic alloy that is highly resistive to wear and has an extremely low coefficient of sliding friction, reaching a record value of 0.04 in unlubricated and 0.02 in lubricated AlMgB14−TiB2 composites. First reported in 1970, BAM has an orthorhombic structure with four icosahedral B12 units per unit cell. This ultrahard material has a coefficient of thermal expansion comparable to that of other widely used materials such as steel and concrete.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).

Ruthenium borides are compounds of ruthenium and boron. Their most remarkable property is potentially high hardness. Vickers hardness HV = 50 GPa was reported for thin films composed of RuB2 and Ru2B3 phases. This value is significantly higher than those of bulk RuB2 or Ru2B3, but it has to be confirmed independently, as measurements on superhard materials are intrinsically difficult. For example, note that the initial report on extreme hardness of related material rhenium diboride was probably too optimistic.

Tantalum borides are compounds of tantalum and boron most remarkable for their extreme hardness.

Tungsten borides are compounds of tungsten and boron. Their most remarkable property is high hardness. The Vickers hardness of WB or WB2 crystals is ~20 GPa and that of WB4 is ~30 GPa for loads exceeding 3 N.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.
Francis Pettit Bundy was an American physicist, known as a member of General Electric's team of researchers that in December 1954 created diamond chips by applying ultra high pressure to graphite with iron sulfide as a catalyst.










