
Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

The trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−. However, the term can also be used to refer to derivatives having substituents on the pyrazolyl rings. This class of compounds belongs to the family of ligands called scorpionate ligands.

In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.

Triiron dodecarbonyl is the organoiron compound with the formula Fe3(CO)12. It is a dark green solid that sublimes under vacuum. It is soluble in nonpolar organic solvents to give intensely green solutions. Most low-nuclearity clusters are pale yellow or orange. Hot solutions of Fe3(CO)12 decompose to an iron mirror, which can be pyrophoric in air.The solid decomposes slowly in air, and thus samples are typically stored cold under an inert atmosphere. It is a more reactive source of iron(0) than iron pentacarbonyl.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.

Decacarbonyldihydridotriosmium is an organoosmium compound with the formula H2Os3(CO)10. This purple-violet crystalline air-stable cluster is noteworthy because it is electron-deficient and hence adds a variety of substrates.
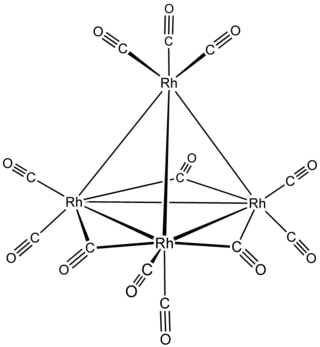
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic synthesis.

Hexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh6(CO)16. It exists as purple-brown crystals that are slightly soluble in dichloromethane and chloroform. It is the principal binary carbonyl of rhodium.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Ruthenium pentacarbonyl is the organoruthenium compound with the formula Ru(CO)5. It is a colorless, light-sensitive liquid that readily decarbonylates upon standing at room temperature. It is of academic interest as an intermediate for the synthesis of metal carbonyl complexes.

Methylidynetricobaltnonacarbonyl is the organocobalt compound with the formula HCCo3(CO)9. It is a metal carbonyl cluster that contains the methylidyne ligand. The compound has C3v point group symmetry. It is a purple, air-stable solid that is soluble in some organic solvents, but not in water.

Tricarbonylbis(triphenylphosphine)iron(0) is a coordination complex with the formula Fe(CO)3(PPh3)2 (Ph = C6H5). A yellow solid, this complex is derived from iron pentacarbonyl by replacement of two carbonyl ligands by triphenylphosphine (PPh3).

Osmium pentacarbonyl is the organoosmium compound with the formula Os(CO)5. It is the simplest isolatable carbonyl complex of osmium. Osmium pentacarbonyl is a colorless volatile liquid that is obtained by treating solid triosmium dodecacarbonyl under 200 atmospheres of carbon monoxide at 280-290 °C. In contrast, also at 200 atm of CO, solid Ru3(CO)12 converts to Ru(CO)5 at milder temperature of 160 °C.
In organometallic chemistry, (diene)iron tricarbonyl describes a diverse family of related coordination complexes consisting of a diene ligand coordinated to a Fe(CO)3 center. Often the diene is conjugated, e.g., butadiene, but the family includes nonconjugated dienes as well. The compounds are yellow, air-stable, often low-melting, and soluble in hydrocarbon solvents. The motif is so robust that even unstable dienes form easily characterized derivatives, such as norbornadienone and cyclobutadiene.





















