 | |
| Names | |
|---|---|
| Preferred IUPAC name Osmocene [1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.687 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
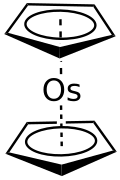
| C10H10Os | |
| Molar mass | 320.42 g·mol−1 |
| Appearance | white solid |
| Melting point | 234 °C |
| Boiling point | 298 °C |
| Structure [2] | |
| orthorhombic | |
| Pnma, No. 62 | |
| D5h | |
| Related compounds | |
Related compounds | ferrocene, ruthenocene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Osmocene is an organoosmium compound found as a white solid. It is a metallocene with the formula Os(C5H5)2.