 | |
 | |
| Names | |
|---|---|
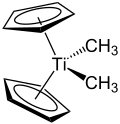
| IUPAC name Bis(η5-cyclopentadienyl)dimethyltitanium | |
| Other names Dimethyltitanocene | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.204.841 |
| EC Number |
|
PubChem CID | |
| |
| |
| Properties | |
| C12H16Ti | |
| Molar mass | 208.13 g/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant, incompatible with water and oxidizing agents |
| GHS labelling: | |
   | |
| Danger | |
| H225, H304, H315, H319, H332, H360, H370, H372 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
The Petasis reagent, named after Nicos A. Petasis, is an organotitanium compound with the formula Cp2Ti(CH3)2. [1] It is an orange-colored solid.

