
An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Titanium isopropoxide, also commonly referred to as titanium tetraisopropoxide or TTIP, is a chemical compound with the formula Ti{OCH(CH3)2}4. This alkoxide of titanium(IV) is used in organic synthesis and materials science. It is a diamagnetic tetrahedral molecule. Titanium isopropoxide is a component of the Sharpless epoxidation, a method for the synthesis of chiral epoxides.

Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.
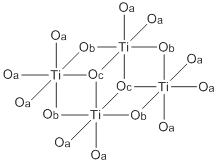
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Its structure is more complex than suggested by its empirical formula. Like other alkoxides of titanium(IV) and zirconium(IV), it finds used in organic synthesis and materials science.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.

Ruthenium(III) acetate, commonly known as basic ruthenium acetate, describes a family of salts where the cation has the formula [Ru3O(O2CCH3)6(OH2)3]+. A representative derivative is the dihydrate of the tetrafluoroborate salt [Ru3O(O2CCH3)6(OH2)3]BF4(H2O)2, which is the source of the data in the table above. This and related salts are forest green, air-stable solids that are soluble in alcohols.
The nickel organic acid salts are organic acid salts of nickel. In many of these the ionised organic acid acts as a ligand.

Potassium octacyanomolybdate(IV) is the inorganic salt with the formula K4[Mo(CN)8]. A yellow light-sensitive solid, it is the potassium salt of the cyanometalate with the coordination number eight. The complex anion consists of a Mo(IV) center bound to eight cyanide ligands resulting in an overall charge of −4, which is balanced with four potassium cations. The salt is often prepared as its dihydrate K4[Mo(CN)8].(H2O)2.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3 as well as at least one hydrate.
Carboxylate–based metal–organic frameworks are metal–organic frameworks that are based on organic molecules comprising carboxylate functional groups.

Hexafluorotitanic acid (systematically named oxonium hexafluoridotitanate(2-)) is an inorganic compound with the chemical formula (H3O)(H5O2)[TiF6]. According to X-ray crystallography, the salt consists of [TiF6]2- octahedral and two kinds of oxonium cations, (H3O)+ and (H5O2)+.












