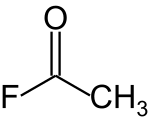
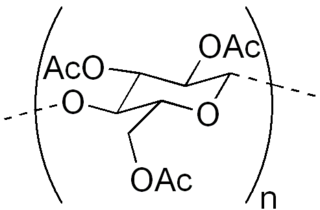In organic chemistry, acetyl is a functional group with the chemical formula −COCH3 and the structure −C(=O)−CH3. It is sometimes represented by the symbol Ac. In IUPAC nomenclature, acetyl is called ethanoyl.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
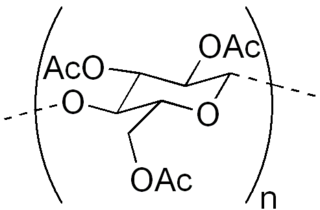
Cellulose triacetate, triacetate, CTA or TAC is a chemical compound produced from cellulose and a source of acetate esters, typically acetic anhydride. Triacetate is commonly used for the creation of fibres and film base. It is chemically similar to cellulose acetate. Its distinguishing characteristic is that in triacetate, at least "92 percent of the hydroxyl groups are acetylated." During the manufacture of triacetate, the cellulose is completely acetylated; whereas in normal cellulose acetate or cellulose diacetate, it is only partially acetylated. Triacetate is significantly more heat resistant than cellulose acetate.
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemicals Ltd, which is more economical and environmentally friendly.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a haloacetic acid, with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms. It is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, Ka, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes.

Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium.
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale than any other acid halide. Specifically, it is generated by the carbonylation of methyl iodide in the Cativa and Monsanto processes, which are the main industrial processes that generate acetic acid. It is also an intermediate in the production of acetic anhydride from methyl acetate.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.

Acetic formic anhydride is an organic compound with the chemical formula C
3H
4O
3, which can be viewed as the mixed anhydride of acetic acid and formic acid. It is used on a laboratory-scale as a formylating agent.
Actinium(III) oxide is a chemical compound containing the rare radioactive element actinium. It has the formula Ac2O3. It is similar to its corresponding lanthanum compound, lanthanum(III) oxide, and contains actinium in the oxidation state +3. Actinium oxide is not to be confused with Ac2O (acetic anhydride), where Ac is an abbreviation for acetyl instead of the symbol of the element actinium.

In organic chemistry, a nitrate ester is an organic functional group with the formula R−ONO2, where R stands for any organyl group. They are the esters of nitric acid and alcohols. A well-known example is nitroglycerin, which is not a nitro compound, despite its name.

Acetyl nitrate is the organic compound with the formula CH3C(O)ONO2. It is classified as the mixed anhydride of nitric and acetic acids. It is a colorless explosive liquid that fumes in moist air.
Aluminium triacetate, formally named aluminium acetate, is a chemical compound with composition Al(CH
3CO
2)
3. Under standard conditions it appears as a white, water-soluble solid that decomposes on heating at around 200 °C. The triacetate hydrolyses to a mixture of basic hydroxide / acetate salts, and multiple species co-exist in chemical equilibrium, particularly in aqueous solutions of the acetate ion; the name aluminium acetate is commonly used for this mixed system.
Aluminium sulfacetate is a mixture of aluminium salts dissolved in water with formula Al
2SO
4(CH
3CO
2)
4.
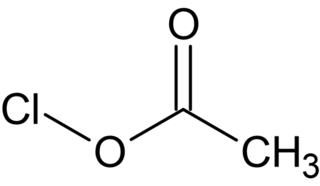
Acetyl hypochlorite, also known as chlorine acetate, is a chemical compound with the formula CH3COOCl. It is a photosensitive colorless liquid that is a short lived intermediate in the Hunsdiecker reaction.