
An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.

Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
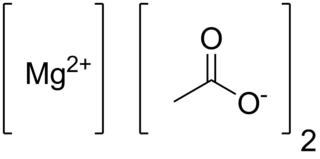
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Barium acetate (Ba(C2H3O2)2) is the salt of barium(II) and acetic acid. Barium acetate is toxic to humans, but it has use in chemistry and manufacturing.

Aluminium monoacetate, also known as dibasic aluminium acetate, and formally named dihydroxy aluminium acetate, is a salt of aluminium with acetic acid. It has the formula Al(OH)2(CH3COO), with aluminium in an oxidation state of +3, and appears under standard conditions as a white solid powder.

Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3.

Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble in water but soluble in concentrated acids.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Ytterbium(III) acetate is an inorganic salt of ytterbium and acetic acid, with a chemical formula of Yb(CH3COO)3. It has colorless crystals that are soluble in water and can form hydrates.

Dysprosium acetate is a hypothetical salt of dysprosium and acetate. Its proposed chemical formula is Dy(CH3COO)3.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3 as well as at least one hydrate.

Cerium acetate is an inorganic compound with the chemical formula of Ce(CH3COO)3. It is a white powder that is soluble in water. Its 1.5 hydrate loses water at 133°C to obtain an amorphous anhydrous form, and the amorphous phase changes to crystal at 212°C, and phase changes again at 286°C.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.

Indium acetate is an acetate of indium, with the chemical formula In(CH3COO)3. It is soluble in water, acetic acid and mineral acids. It is the precursor of indium-containing compounds such as the solar cell materials CuInS2 and indium phosphide quantum dots.
Tin(IV) acetate is the acetate salt of tin(IV), with the chemical formula of Sn(CH3COO)4.
















