
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.
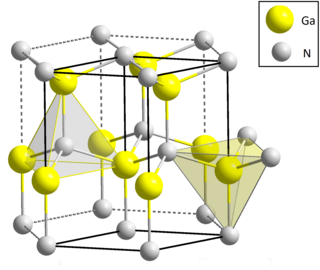
Gallium nitride is a binary III/V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronics, high-power and high-frequency devices. For example, GaN is the substrate that makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency doubling.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.

Silicon nitride is a chemical compound of the elements silicon and nitrogen. Si
3N
4 is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″Silicon nitride″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot H
3PO
4. It is very hard. It has a high thermal stability with strong optical nonlinearities for all-optical applications.

Zinc nitride (Zn3N2) is an inorganic compound of zinc and nitrogen, usually obtained as (blue)grey crystals. It is a semiconductor. In pure form, it has the anti-bixbyite structure.
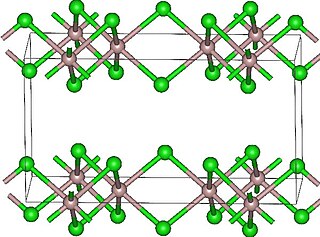
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hygroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.

Lutetium tantalate is a chemical compound of lutetium, tantalum and oxygen with the formula LuTaO4. With a density of 9.81 g/cm3, this mixed oxide compound is the densest known white stable material. (Although thorium dioxide ThO2 is also white and has a higher density of 10 g/cm3, it is radioactively unstable; while not radioactive enough to make it unstable as a material, even its low rate of decay is still too much for certain uses such as phosphors for detecting ionising radiation.) The white color and high density of LuTaO4 make it ideal for phosphor applications, though the high cost of lutetium is a hindrance.
In chemistry, a hydridonitride is a chemical compound that contains both hydride and nitride ions. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and usually contain a larger proportion of metals.

Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.
Lutetium phosphide is an inorganic compound of lutetium and phosphorus with the chemical formula LuP. The compound forms dark crystals, does not dissolve in water.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.

Dypsrosium nitride is a binary inorganic compound of dysprosium and nitride with the chemical formula DyN.

Gadolinium(III) nitride is a binary inorganic compound of gadolinium and nitrogen with the chemical formula GdN.
Curium nitride is a binary inorganic compound of curium and nitrogen with the chemical formula CmN.
Terbium nitride is a binary inorganic compound of terbium and nitrogen with the chemical formula TbN.











