 | |
| Names | |
|---|---|
| IUPAC name Zirconium nitride | |
| Other names Zirconium(III) nitride, Nitridozirconium | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.042.864 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| ZrN [1] | |
| Appearance | Yellow-brown crystals |
| Odor | Odorless |
| Density | 7.09 g/cm3 (24 °C) [1] |
| Melting point | 2,952 °C (5,346 °F; 3,225 K) at 760 mmHg [1] |
| Insoluble | |
| Solubility | Soluble in concentrated HF, acids [1] |
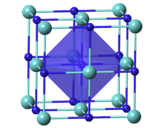
| Structure | |
| Cubic, cF8 [2] | |
| Fm3m, No. 225 [2] | |
α = 90°, β = 90°, γ = 90° | |
| Octahedral [2] | |
| Thermochemistry | |
Heat capacity (C) | 40.442 J/(mol·K) [3] |
Std molar entropy (S⦵298) | 38.83 J/(mol·K) [3] |
Std enthalpy of formation (ΔfH⦵298) | −365.26 kJ/mol [3] |
| Related compounds | |
Related refractory ceramic materials | Tantalum carbide Niobium carbide Zirconium carbide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Zirconium nitride ( Zr N ) is an inorganic compound used in a variety of ways due to its properties.
