 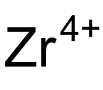 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
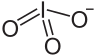
| I4O12Zr | |
| Molar mass | 790.830 g·mol−1 |
| Appearance | white solid |
| Density | 4.99 |
| Structure | |
| tetragonal | |
| P4/n | |
Lattice volume (V) | 526 Å3 |
Formula units (Z) | 2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Zirconium iodate is an inorganic compound with the chemical formula Zr(IO3)4. It can be prepared by reacting sodium iodate and zirconium sulfate tetrahydrate in an aqueous solution. The resulting precipitate is dried and refluxed in concentrated nitric acid. [1] Zirconium iodate trihydrate can be obtained by reacting hydrated zirconium oxide and iodine pentoxide (1.4~3.3% concentration) in water. [2] Its basic salt Zr(OH)n(IO3)4−n is known. [3]