
Zirconium is a chemical element; it has symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, ductile, malleable and corrosion-resistant. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin.

Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound.

Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One of the main uses of zirconium alloys is in nuclear technology, as cladding of fuel rods in nuclear reactors, especially water reactors. A typical composition of nuclear-grade zirconium alloys is more than 95 weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel and other metals, which are added to improve mechanical properties and corrosion resistance.
Zirconium hydride describes an alloy made by combining zirconium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the zirconium atom crystal lattice from sliding past one another. Varying the amount of hydrogen and the form of its presence in the zirconium hydride controls qualities such as the hardness, ductility, and tensile strength of the resulting zirconium hydride. Zirconium hydride with increased hydrogen content can be made harder and stronger than zirconium, but such zirconium hydride is also less ductile than zirconium.

Zirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.
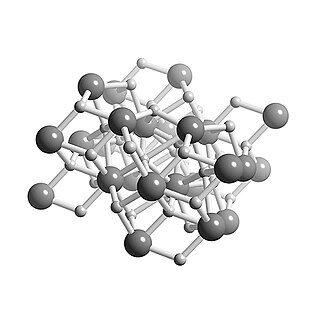
Calcium hydride is the chemical compound with the formula CaH2, an alkaline earth hydride. This grey powder reacts vigorously with water liberating hydrogen gas. CaH2 is thus used as a drying agent, i.e. a desiccant.
Zirconium(IV) bromide is the inorganic compound with the formula ZrBr4. This colourless solid is the principal precursor to other Zr–Br compounds.

Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.

Titanium hydride normally refers to the inorganic compound TiH2 and related nonstoichiometric materials. It is commercially available as a stable grey/black powder, which is used as an additive in the production of Alnico sintered magnets, in the sintering of powdered metals, the production of metal foam, the production of powdered titanium metal and in pyrotechnics.

Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.

Plumbane is an inorganic chemical compound with the chemical formula PbH4. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom. Derivatives of plumbane include lead tetrafluoride, PbF4, and tetraethyllead, (CH3CH2)4Pb.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.
Uranium hydride, also called uranium trihydride (UH3), is an inorganic compound and a hydride of uranium.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.
Scandium trihydride is an unstable molecular chemical compound with the chemical formula ScH3. It has been formed as one of a number of other molecular scandium hydride products at low temperature using laser ablation and identified by infrared spectroscopy. Scandium trihydride has recently been the subject of Dirac–Hartree–Fock relativistic calculation studies, which investigate the stabilities, geometries, and relative energies of hydrides of the formula MH3, MH2, or MH.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.















