
Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Gallium(III) bromide (GaBr3) is a chemical compound, and one of four gallium trihalides.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.
Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.

Gallium trichloride is the chemical compound with the formula GaCl3. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6. It is colourless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.
The thallium halides include monohalides, where thallium has oxidation state +1, trihalides in which thallium generally has oxidation state +3, and some intermediate halides containing thallium with mixed +1 and +3 oxidation states. These materials find use in specialized optical settings, such as focusing elements in research spectrophotometers. Compared to the more common zinc selenide-based optics, materials such as thallium bromoiodide enable transmission at longer wavelengths. In the infrared, this allows for measurements as low as 350 cm−1 (28 μm), whereas zinc selenide is opaque by 21.5 μm, and ZnSe optics are generally only usable to 650 cm−1 (15 μm).
There are three sets of gallium halides, the trihalides where gallium has oxidation state +3, the intermediate halides containing gallium in oxidation states +1, +2 and +3 and some unstable monohalides, where gallium has oxidation state +1.

Niobium(IV) chloride, also known as niobium tetrachloride, is the chemical compound of formula NbCl4. This compound exists as dark violet crystals, is highly sensitive to air and moisture, and disproportiates into niobium(III) chloride and niobium(V) chloride when heated.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.
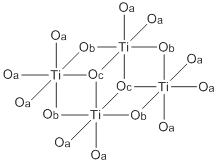
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a colorless liquid that is soluble in organic solvents but hydrolyzes readily. It is sold commercially as a colorless solution. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas.
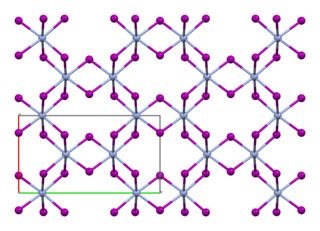
Chromium(III) iodide, also known as chromium triiodide, is an inorganic compound with the formula CrI3. It is a black solid that is used to prepare other chromium iodides.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.

Zirconium(III) bromide is an inorganic compound with the formula ZrBr3.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula HfI3. It is a black solid.














