
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −2.7) and an oxidizing agent.

Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

Iodic acid is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

Periodic acid is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. It can exist in two forms: orthoperiodic acid, with the chemical formula H5IO6, and metaperiodic acid, which has the formula HIO4.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
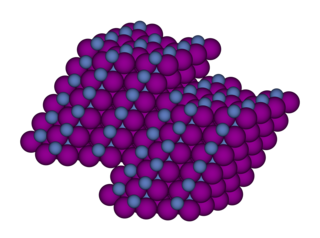
Nickel(II) iodide is an inorganic compound with the formula NiI2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above). This bluish-green colour is typical of hydrated nickel(II) compounds. Nickel iodides find some applications in homogeneous catalysis.

Potassium iodate (KIO3) is an ionic inorganic compound with the formula KIO3. It is a white salt that is soluble in water.

Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.

Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Discovered in 1814 by Gay-Lussac, iodine monochloride is the first interhalogen compound discovered.
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.

Hypoiodous acid is an inorganic compound with the chemical formula HOI, also written as HIO. It forms when an aqueous solution of iodine is treated with mercuric or silver salts. It rapidly decomposes by disproportionation:

Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:

Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.

Germanium(IV) iodide is an inorganic compound with the chemical formula GeI4.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Astatine compounds are compounds that contain the element astatine (At). As this element is very radioactive, few compounds have been studied. Less reactive than iodine, astatine is the least reactive of the halogens. Its compounds have been synthesized in nano-scale amounts and studied as intensively as possible before their radioactive disintegration. The reactions involved have been typically tested with dilute solutions of astatine mixed with larger amounts of iodine. Acting as a carrier, the iodine ensures there is sufficient material for laboratory techniques to work. Like iodine, astatine has been shown to adopt odd-numbered oxidation states ranging from −1 to +7.
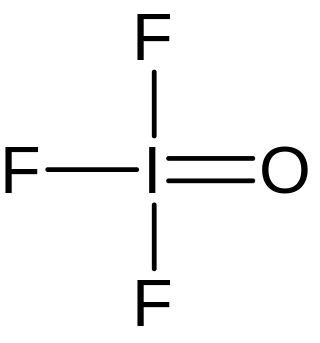
Iodosyl trifluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IOF3.
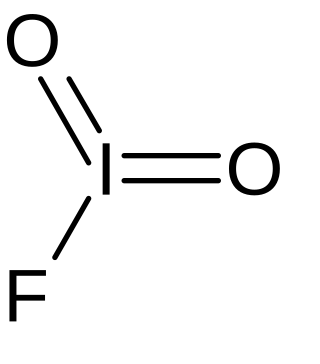
Iodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F. The compound was initially synthesized in 1951.

















