Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, originally found in the gadolinite mine in Ytterby, Sweden, which is the source of the element's name.
The lanthanide or lanthanoid series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals.

Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density and melting and boiling points differ significantly from those of most other lanthanides.

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.

Curium(III) oxide is a compound composed of curium and oxygen with the chemical formula Cm2O3. It is a crystalline solid with a unit cell that contains two curium atoms and three oxygen atoms. The simplest synthesis equation involves the reaction of curium(III) metal with O2−: 2 Cm3+ + 3 O2− ---> Cm2O3. Curium trioxide can exist as five polymorphic forms. Two of the forms exist at extremely high temperatures, making it difficult for experimental studies to be done on the formation of their structures. The three other possible forms which curium sesquioxide can take are the body-centered cubic form, the monoclinic form, and the hexagonal form. Curium(III) oxide is either white or light tan in color and, while insoluble in water, is soluble in inorganic and mineral acids. Its synthesis was first recognized in 1955.
A sesquioxide is an oxide of an element, where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide Al2O3 and phosphorus(III) oxide P4O6 are sesquioxides. Many sesquioxides contain a metal in the +3 oxidation state and the oxide ion O2−, e.g., aluminium oxide Al2O3, lanthanum(III) oxide La2O3 and iron(III) oxide Fe2O3. Sesquioxides of iron and aluminium are found in soil. The alkali metal sesquioxides are exceptions because they contain both peroxide O2−2 and superoxide O−2 ions, e.g., rubidium sesquioxide Rb4O6 is formulated (Rb+)4(O2−2)(O−2)2. Sesquioxides of metalloids and nonmetals are exceptions too, e.g. boron trioxide B2O3, dinitrogen trioxide N2O3 and phosphorus(III) oxide P4O6.

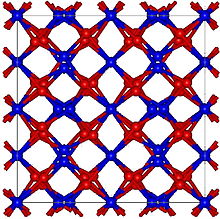
Gadolinium(III) oxide (archaically gadolinia) is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the rare-earth element gadolinium, derivatives of which are potential contrast agents for magnetic resonance imaging.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.

Terbium(III) bromide (TbBr3) is a crystalline chemical compound.

Manganese(III) oxide is a chemical compound with the formula Mn2O3. It occurs in nature as the mineral bixbyite (recently changed to bixbyite-(Mn)) and is used in the production of ferrites and thermistors.

Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust.

Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish-blue hexagonal crystals. The rare-earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.

Terbium(III) nitrate is an inorganic chemical compound, a salt of terbium and nitric acid, with the formula Tb(NO3)3. The hexahydrate crystallizes as triclinic colorless crystals with the formula [Tb(NO3)3(H2O)4]·2H2O. It can be used to synthesize materials with green emission.

Americium(III) oxide or americium sesquioxide is an oxide of the element americium. It has the empirical formula Am2O3. Since all isotopes of americium are only artificially produced, americium (III) oxide has no natural occurrence. The colour depends on the crystal structure, of which there are more than one. It is soluble in acids.

Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).