
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide. Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.

Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite, or sometimes black copper. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters.

Cobalt(II) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid compound which is antiferromagnetic at low temperatures (TN=37.7 K) The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate red orthogonal crystal, (CoF2·4H2O). CoF2 is used in oxygen-sensitive fields, namely metal production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can be dissolved in warm mineral acid, and will decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoF2·2H2O and tri-hydrate CoF2·3H2O forms of the compound. The hydrate will also decompose with heat.

Cadmium fluoride (CdF2) is a mostly water-insoluble source of cadmium used in oxygen-sensitive applications, such as the production of metallic alloys. In extremely low concentrations (ppm), this and other fluoride compounds are used in limited medical treatment protocols. Fluoride compounds also have significant uses in synthetic organic chemistry. The standard enthalpy has been found to be -167.39 kcal. mole−1 and the Gibbs energy of formation has been found to be -155.4 kcal. mole−1, and the heat of sublimation was determined to be 76 kcal. mole−1.
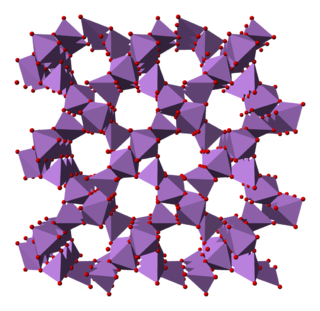
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Aluminium nitrate is a white, water-soluble salt of aluminium and nitric acid, most commonly existing as the crystalline hydrate, aluminium nitrate nonahydrate, Al(NO3)3·9H2O.

Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.

Antimony trichloride is the chemical compound with the formula SbCl3. It is a soft colorless solid with a pungent odor and was known to alchemists as butter of antimony.

Samarium(III) oxide (Sm2O3) is a chemical compound. Samarium oxide readily forms on the surface of samarium metal under humid conditions or temperatures in excess of 150°C in dry air. Similar to rust on metallic iron, this oxide layer spalls off the surface of the metal, exposing more metal to continue the reaction. The oxide is commonly white to off yellow in color and is often encountered as a highly fine dust like powder.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.
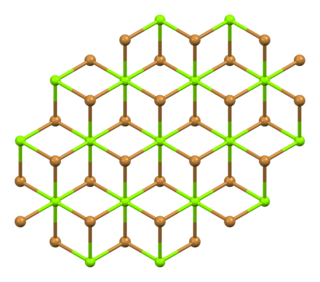
Magnesium bromide are inorganic compounds with the chemical formula MgBr2(H2O)x, where x can range from 0 to 9. They are all white deliquescent solids. Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. Like other inorganic arsenic compounds, it is highly toxic.

Beryllium sulfate normally encountered as the tetrahydrate, [Be(H2O)4]SO4 is a white crystalline solid. It was first isolated in 1815 by Jons Jakob Berzelius. Beryllium sulfate may be prepared by treating an aqueous solution of many beryllium salts with sulfuric acid, followed by evaporation of the solution and crystallization. The hydrated product may be converted to anhydrous salt by heating at 400 °C.

Mercury(I) nitrate is an inorganic compound, a salt of mercury and nitric acid with the formula Hg2(NO3)2. A yellow solid, the compound is used as a precursor to other Hg22+ complexes. The structure of the hydrate has been determined by X-ray crystallography. It consists of a [H2O-Hg-Hg-OH2]2+ center, with a Hg-Hg distance of 254 pm.



























