
Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.
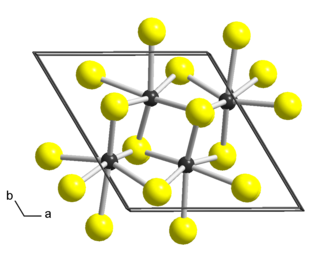
Rhenium disulfide is an inorganic compound of rhenium and sulfur with the formula ReS2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
An oxyhydride is a mixed anion compound containing both oxide O2− and hydride ions H−. These compounds may be unexpected as the hydrogen and oxygen could be expected to react to form water. But if the metals making up the cations are electropositive enough, and the conditions are reducing enough, solid materials can be made that combine hydrogen and oxygen in the negative ion role.
Carbohydrides are solid compounds in one phase composed of a metal with carbon and hydrogen in the form of carbide and hydride ions. The term carbohydride can also refer to a hydrocarbon.
In chemistry, a hydridonitride is a chemical compound that contains both hydride and nitride ions. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and usually contain a larger proportion of metals.
The telluride bromides are chemical compounds that contain both telluride ions (Te2−) and bromide ions (Br−). They are in the class of mixed anion compounds or chalcogenide halides.
The telluride oxides or oxytellurides are double salts that contain both telluride and oxide anions. They are in the class of mixed anion compounds.
The telluride iodides are chemical compounds that contain both telluride ions (Te2−) and iodide ions (I−). They are in the class of mixed anion compounds or chalcogenide halides.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.
Borate sulfides are chemical mixed anion compounds that contain any kind of borate and sulfide ions. They are distinct from thioborates in which sulfur atoms replace oxygen in borates. There are also analogous borate selenides, with selenium ions instead of sulfur.
Antimony phosphate, is a chemical compound of antimony and phosphate with formula SbPO4. Antimony is in the form Sb(III) with +3 oxidation state. Antimony atoms have a lone pair of electrons.
Silicide carbides or carbide silicides are compounds containing anions composed of silicide (Si4−) and carbide (C4−) or clusters therof. They can be considered as mixed anion compounds or intermetallic compounds, as silicon could be considered as a semimetal.
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.
Neodymium(III) hydride is an inorganic compound composed of neodymium and hydrogen with a chemical formula NdH3. In this compound, the neodymium atom is in the +3 oxidation state and the hydrogen atoms are -1. It is highly reactive.
Phosphide iodides or iodide phosphides are compounds containing anions composed of iodide (I−) and phosphide (P3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the phosphide chlorides, arsenide iodides antimonide iodides and phosphide bromides.
Arsenide tellurides or telluride arsenides are compounds containing anions composed of telluride (Te2−) and arsenide (As3−). They can be considered as mixed anion compounds. Related compounds include the arsenide sulfides, arsenide selenides, antimonide tellurides, and phosphide tellurides. Some are in the category of arsenopyrite-type compounds with As:Te of 1:1. Yet others are layered with As:Te of 1:2.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3 as well as at least one hydrate.
Bromoantimonates are compounds containing anions composed of bromide (Br−) and antimony (Sb). They can be considered as double bromides, metallohalides or halometallates. They are in the category of halopnictates. Related compounds include the bromobismuthates, iodoantimonates, bromophosphates, and bromoarsenates.
Thulium(III) selenide is an inorganic compound with the chemical formula Tm2Se3.
Erbium(III) selenide is an inorganic compound with a chemical formula of Er2Se3.


