
Antimony is a chemical element; it has symbol Sb (from Latin stibium) and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio.

Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy.

Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.

A neodymium magnet (also known as NdFeB, NIB or Neo magnet) is a permanent magnet made from an alloy of neodymium, iron, and boron to form the Nd2Fe14B tetragonal crystalline structure. They are the most widely used type of rare-earth magnet.
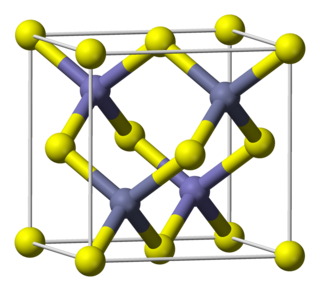
Indium antimonide (InSb) is a crystalline compound made from the elements indium (In) and antimony (Sb). It is a narrow-gap semiconductor material from the III-V group used in infrared detectors, including thermal imaging cameras, FLIR systems, infrared homing missile guidance systems, and in infrared astronomy. Indium antimonide detectors are sensitive to infrared wavelengths between 1 and 5 μm.

Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.
Naturally occurring dysprosium (66Dy) is composed of 7 stable isotopes, 156Dy, 158Dy, 160Dy, 161Dy, 162Dy, 163Dy and 164Dy, with 164Dy being the most abundant. Twenty-nine radioisotopes have been characterized, with the most stable being 154Dy with a half-life of 1.4 million years, 159Dy with a half-life of 144.4 days, and 166Dy with a half-life of 81.6 hours. All of the remaining radioactive isotopes have half-lives that are less than 10 hours, and the majority of these have half-lives that are less than 30 seconds. This element also has 12 meta states, with the most stable being 165mDy, 147mDy and 145mDy.

Gallium antimonide (GaSb) is a semiconducting compound of gallium and antimony of the III-V family. It has a room temperature lattice constant of about 0.610 nm. It has a room temperature direct bandgap of approximately 0.73 eV.

Yttrium(III) antimonide (YSb) is an inorganic chemical compound.
Indium arsenide antimonide phosphide is a semiconductor material.

Dysprosium(III) hydroxide is an inorganic compound with the chemical formula Dy(OH)3.
Dysprosium(II) chloride (DyCl2), also known as dysprosium dichloride, is an ionic chemical compound of dysprosium and chlorine. This salt is a reduced compound, as the normal oxidation state of dysprosium in dysprosium compounds is +3.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.

Dysprosium phosphide is an inorganic compound of dysprosium and phosphorus with the chemical formula DyP.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
An iodide nitride is a mixed anion compound containing both iodide (I−) and nitride ions (N3−). Another name is metalloiodonitrides. They are a subclass of halide nitrides or pnictide halides. Some different kinds include ionic alkali or alkaline earth salts, small clusters where metal atoms surround a nitrogen atom, layered group 4 element 2-dimensional structures, and transition metal nitrido complexes counter-balanced with iodide ions. There is also a family with rare earth elements and nitrogen and sulfur in a cluster.
Antimonide iodides or iodide antimonides are compounds containing anions composed of iodide (I−) and antimonide (Sb3−). They can be considered as mixed anion compounds. They are in the category of pnictide halides. Related compounds include the antimonide chlorides, antimonide bromides, phosphide iodides, and arsenide iodides.

Dysprosium arsenide is a binary inorganic compound of dysprosium and arsenide with the chemical formula DyAs.

Dysprosium monosulfide is a binary inorganic compound of dysprosium and sulfur with the chemical formula DyS.
Dysprosium(III) sulfide is a binary inorganic compound of dysprosium and sulfur with the chemical formula Dy2S3.












