Related Research Articles
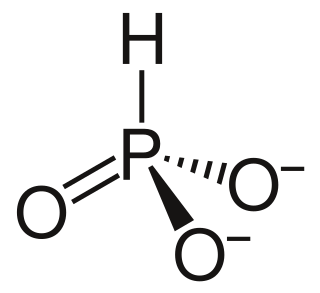
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.
In chemistry, the term stannate or tinnate refers to compounds of tin (Sn). Stannic acid (Sn(OH)4), the formal precursor to stannates, does not exist and is actually a hydrate of SnO2. The term is also used in naming conventions as a suffix; for example the hexachlorostannate ion is SnCl2−
6.

In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.

In chemistry, tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.

Telluric acid, or more accurately orthotelluric acid, is a chemical compound with the formula Te(OH)6, often written as H6TeO6. It is a white crystalline solid made up of octahedral Te(OH)6 molecules which persist in aqueous solution. In the solid state, there are two forms, rhombohedral and monoclinic, and both contain octahedral Te(OH)6 molecules, containing one hexavalent tellurium (Te) atom in the +6 oxidation state, attached to six hydroxyl (–OH) groups, thus, it can be called tellurium(VI) hydroxide. Telluric acid is a weak acid which is dibasic, forming tellurate salts with strong bases and hydrogen tellurate salts with weaker bases or upon hydrolysis of tellurates in water. It is used as tellurium-source in the synthesis of oxidation catalysts.

Arsenic acid or arsoric acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (2H3AsO4·H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.
In chemistry, an arsenite is a chemical compound containing an arsenic oxyanion where arsenic has oxidation state +3. Note that in fields that commonly deal with groundwater chemistry, arsenite is used generically to identify soluble AsIII anions. IUPAC have recommended that arsenite compounds are to be named as arsenate(III), for example ortho-arsenite is called trioxidoarsenate(III). Ortho-arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent.
Chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.
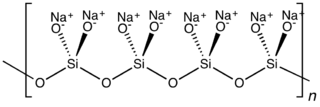
Sodium metasilicate is the chemical substance with formula Na
2SiO
3, which is the main component of commercial sodium silicate solutions. It is an ionic compound consisting of sodium cations Na+
and the polymeric metasilicate anions [–SiO2−
3–]n. It is a colorless crystalline hygroscopic and deliquescent solid, soluble in water but not in alcohols.

Antimony pentoxide (molecular formula: Sb2O5) is a chemical compound of antimony and oxygen. It contains antimony in the +5 oxidation state.
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of Nomenclature of Inorganic Chemistry. It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC).

In chemistry, germanate is a compound containing an oxyanion of germanium. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central germanium atom, for example potassium hexafluorogermanate, K2GeF6.
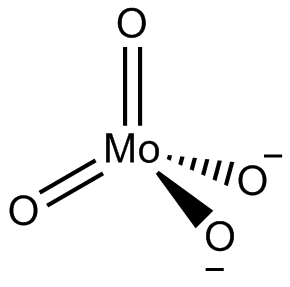
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of +6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
A tellurite tellurate is chemical compound or salt that contains tellurite and tellurate anions [TeO3]2- [TeO4 ]2-. These are mixed anion compounds, meaning the compounds are cations that contain one or more anions. Some have third anions. Environmentally, tellurite [TeO3]2- is the more abundant anion due to tellurate's [TeO4 ]2- low solubility limiting its concentration in biospheric waters. Another way to refer to the anions is tellurium's oxyanions, which happen to be relatively stable.
References
- 1 2 3 4 5 6 7 Wells A.F. (1984) Structural Inorganic Chemistry 5th edition, Oxford Science Publications ISBN 0-19-855370-6
- ↑ Nomenclature of Inorganic Chemistry—IUPAC Recommendations 2005 Red Book 2005.pdf
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.