
Gallium is a chemical element; it has the symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, gallium is in group 13 of the periodic table and is similar to the other metals of the group.

Aluminium gallium arsenide (AlxGa1−xAs) is a semiconductor material with very nearly the same lattice constant as GaAs, but a larger bandgap. The x in the formula above is a number between 0 and 1 - this indicates an arbitrary alloy between GaAs and AlAs.

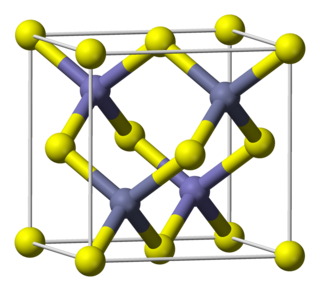
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.

A photocathode is a surface engineered to convert light (photons) into electrons using the photoelectric effect. Photocathodes are important in accelerator physics where they are utilised in a photoinjector to generate high brightness electron beams. Electron beams generated with photocathodes are commonly used for free electron lasers and for ultrafast electron diffraction. Photocathodes are also commonly used as the negatively charged electrode in a light detection device such as a photomultiplier, phototube and image intensifier.
Indium antimonide (InSb) is a crystalline compound made from the elements indium (In) and antimony (Sb). It is a narrow-gap semiconductor material from the III-V group used in infrared detectors, including thermal imaging cameras, FLIR systems, infrared homing missile guidance systems, and in infrared astronomy. Indium antimonide detectors are sensitive to infrared wavelengths between 1 and 5 μm.
Indium gallium arsenide (InGaAs) is a ternary alloy of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are group III elements of the periodic table while arsenic is a group V element. Alloys made of these chemical groups are referred to as "III-V" compounds. InGaAs has properties intermediate between those of GaAs and InAs. InGaAs is a room-temperature semiconductor with applications in electronics and photonics.

An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).

Aluminium antimonide (AlSb) is a semiconductor of the group III-V family containing aluminium and antimony. The lattice constant is 0.61 nm. The indirect bandgap is approximately 1.6 eV at 300 K, whereas the direct band gap is 2.22 eV.
Aluminium indium arsenide, also indium aluminium arsenide or AlInAs (AlxIn1−xAs), is a ternary III-V semiconductor compound with very nearly the same lattice constant as InGaAs, but a larger bandgap. It can be considered as an alloy between aluminium arsenide (AlAs) and indium arsenide (InAs). AlInAs refers generally to any composition of the alloy.
Gallium indium arsenide antimonide phosphide is a semiconductor material.
Indium arsenide antimonide phosphide is a semiconductor material.
Morton B. Panish is an American physical chemist who, with Izuo Hayashi, developed a room-temperature continuous wave semiconductor laser in 1970. For this achievement he shared the Kyoto Prize in Advanced Technology in 2001.

Rubin Braunstein (1922–2018) was an American physicist and educator. In 1955 he published the first measurements of light emission by semiconductor diodes made from crystals of gallium arsenide (GaAs), gallium antimonide (GaSb), and indium phosphide (InP). GaAs, GaSb, and InP are examples of III-V semiconductors. The III-V semiconductors absorb and emit light much more strongly than silicon, which is the best-known semiconductor. Braunstein's devices are the forerunners of contemporary LED lighting and semiconductor lasers, which typically employ III-V semiconductors. The 2000 and 2014 Nobel Prizes in Physics were awarded for further advances in closely related fields.
Aluminium indium antimonide, also known as indium aluminium antimonide or AlInSb (AlxIn1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium antimonide and indium antimonide. The alloy can contain any ratio between aluminium and indium. AlInSb refers generally to any composition of the alloy.
Aluminium gallium antimonide, also known as gallium aluminium antimonide or AlGaSb (AlxGa1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium antimonide and gallium antimonide. The alloy can contain any ratio between aluminium and gallium. AlGaSb refers generally to any composition of the alloy.
Gallium arsenide antimonide, also known as gallium antimonide arsenide or GaAsSb, is a ternary III-V semiconductor compound; x indicates the fractions of arsenic and antimony in the alloy. GaAsSb refers generally to any composition of the alloy. It is an alloy of gallium arsenide (GaAs) and gallium antimonide (GaSb).
Indium arsenide antimonide, also known as indium antimonide arsenide or InAsSb (InAs1-xSbx), is a ternary III-V semiconductor compound. It can be considered as an alloy between indium arsenide (InAs) and indium antimonide (InSb). The alloy can contain any ratio between arsenic and antimony. InAsSb refers generally to any composition of the alloy.
Gallium indium antimonide, also known as indium gallium antimonide, GaInSb, or InGaSb (GaxIn1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between gallium antimonide and indium antimonide. The alloy can contain any ratio between gallium and indium. GaInSb refers generally to any composition of the alloy.
Aluminium arsenide antimonide, or AlAsSb (AlAs1-xSbx), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium arsenide and aluminium antimonide. The alloy can contain any ratio between arsenic and antimony. AlAsSb refers generally to any composition of the alloy.









