
In the physical sciences, a phase is a region of space, throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.

Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from the Greek word πιέζειν; piezein, which means to squeeze or press, and ἤλεκτρον ēlektron, which means amber, an ancient source of electric charge.

A crystal oscillator is an electronic oscillator circuit that uses the mechanical resonance of a vibrating crystal of piezoelectric material to create an electrical signal with a constant frequency. This frequency is often used to keep track of time, as in quartz wristwatches, to provide a stable clock signal for digital integrated circuits, and to stabilize frequencies for radio transmitters and receivers. The most common type of piezoelectric resonator used is a quartz crystal, so oscillator circuits incorporating them became known as crystal oscillators. However, other piezoelectric materials including polycrystalline ceramics are used in similar circuits.
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.

In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
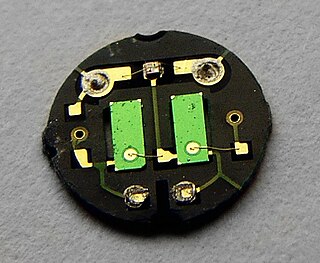
Pyroelectricity is a property of certain crystals which are naturally electrically polarized and as a result contain large electric fields. Pyroelectricity can be described as the ability of certain materials to generate a temporary voltage when they are heated or cooled. The change in temperature modifies the positions of the atoms slightly within the crystal structure, such that the polarization of the material changes. This polarization change gives rise to a voltage across the crystal. If the temperature stays constant at its new value, the pyroelectric voltage gradually disappears due to leakage current. The leakage can be due to electrons moving through the crystal, ions moving through the air, or current leaking through a voltmeter attached across the crystal.

Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It is used in dentistry as a component of alginate impression materials as well as for making models of teeth.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
A quartz crystal microbalance (QCM) measures a mass variation per unit area by measuring the change in frequency of a quartz crystal resonator. The resonance is disturbed by the addition or removal of a small mass due to oxide growth/decay or film deposition at the surface of the acoustic resonator. The QCM can be used under vacuum, in gas phase and more recently in liquid environments. It is useful for monitoring the rate of deposition in thin film deposition systems under vacuum. In liquid, it is highly effective at determining the affinity of molecules to surfaces functionalized with recognition sites. Larger entities such as viruses or polymers are investigated as well. QCM has also been used to investigate interactions between biomolecules. Frequency measurements are easily made to high precision ; hence, it is easy to measure mass densities down to a level of below 1 μg/cm2. In addition to measuring the frequency, the dissipation factor is often measured to help analysis. The dissipation factor is the inverse quality factor of the resonance, Q−1 = w/fr ; it quantifies the damping in the system and is related to the sample's viscoelastic properties.

Lanthanum gallium silicate (referred to as LGS in this article), also known as langasite, has a chemical formula of the form A3BC3D2O14, where A, B, C and D indicate particular cation sites. A is a decahedral (Thomson cube) site coordinated by 8 oxygen atoms. B is octahedral site coordinated by 6 oxygen atoms, and C and D are tetrahedral sites coordinated by 4 oxygen atoms. In this material, lanthanum occupied the A-sites, gallium the B, C and half of D-sites, and, silicon the other half of D-sites.

A piezoelectric sensor is a device that uses the piezoelectric effect to measure changes in pressure, acceleration, temperature, strain, or force by converting them to an electrical charge. The prefix piezo- is Greek for 'press' or 'squeeze'.
A thin-film bulk acoustic resonator is a device consisting of a piezoelectric material manufactured by thin film methods between two conductive – typically metallic – electrodes and acoustically isolated from the surrounding medium. The operation is based on the piezoelectricity of the piezolayer between the electrodes.

Gallium(III) trioxide is an inorganic compound with the formula Ga2O3. It exists as several polymorphs, all of which are white, water-insoluble solids. Although no commercial applications exist, Ga2O3 is an intermediate in the purification of gallium, which is consumed almost exclusively as gallium arsenide. The thermal conductivity of β-Ga2O3 is at least one order of magnitude lower than the other wide bandgap semiconductors, such as GaN and SiC. It is further reduced for related nanostructures which are usually used in electronic devices. Heterogeneous integration with high thermal conductivity substrates such as diamond and SiC helps heat dissipation of β-Ga2O3 electronics.
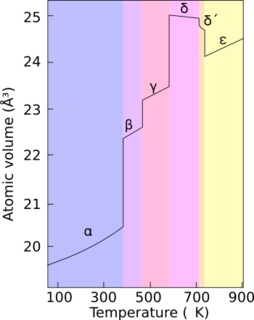
Plutonium occurs in a variety of allotropes, even at ambient pressure. These allotropes differ widely in crystal structure and density; the α and δ allotropes differ in density by more than 25% at constant pressure.

Gallium(II) selenide (GaSe) is a chemical compound. It has a hexagonal layer structure, similar to that of GaS. It is a photoconductor, a second harmonic generation crystal in nonlinear optics, and has been used as a far-infrared conversion material at 14–31 THz and above.

Ferroelectric polymers are a group of crystalline polar polymers that are also ferroelectric, meaning that they maintain a permanent electric polarization that can be reversed, or switched, in an external electric field.
Plutonium–gallium alloy (Pu–Ga) is an alloy of plutonium and gallium, used in nuclear weapon pits, the component of a nuclear weapon where the fission chain reaction is started. This alloy was developed during the Manhattan Project.
Microelectromechanical system oscillators Devices that generate highly stable reference frequencies to measure time. The core technologies used in MEMS oscillators have been in development since the mid-1960s, but have only been sufficiently advanced for commercial applications since 2006. MEMS oscillators incorporate MEMS resonators, which are microelectromechanical structures that define stable frequencies. MEMS clock generators are MEMS timing devices with multiple outputs for systems that need more than a single reference frequency. MEMS oscillators are a valid alternative to older, more established quartz crystal oscillators, offering better resilience against vibration and mechanical shock, and reliability with respect to temperature variation.
The glass forming ability of gallium(III) sulfide and lanthanum sulfide was discovered in 1976 by Loireau-Lozac’h, Guittard, and Flahut. This family of chalcogenide glasses, referred to as gallium lanthanum sulfide (Ga-La-S) glasses, have a wide region of glass formation centred about the 70Ga2S3:30La2S3 composition and can readily accept other modifiers into their structure. This means that Ga-La-S can be compositionally adjusted to give a wide variety of optical and physical properties. Optically, Ga-La-S has a high refractive index, a transmission window covering most of the visible wavelengths and extending to about 10 µm and a low maximum phonon energy, approx. 450 cm−1. Thermally, the refractive index of Ga-La-S glasses has a strong temperature dependence and low thermal conductivity, which results in strong thermal lensing. However, the high glass transition temperature of Ga-La-S makes it resistant to thermal damage, it has good chemical durability and unlike many chalcogenides which are based on arsenic, its glass components are non-toxic. A clear advantage over other chalcogenides is its high lanthanum content which allows excellent rare-earth solubility and dispersion of the ions in the glass matrix for active devices. Ga-La-S can exist in both glassy and crystalline phases, in a glassy phase, it is a semiconductor with a bandgap of 2.6 eV corresponding to a wavelength of 475 nm; consequently Ga-La-S glass takes a deep orange colour. As with all chalcogenides the phase of the bulk is determined by two key factors; the material composition and the rate at which the molten material is cooled. These variables can be controlled to manipulate the final phase of the material.
Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.