Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially. While classified as a strong base, lithium hydroxide is the weakest known alkali metal hydroxide.

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless solids. They are used mainly as superphosphate fertilizers and are also popular leavening agents.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.

Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, an important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.

Lithium peroxide is the inorganic compound with the formula Li2O2. Lithium peroxide is a white solid, and unlike most other alkali metal peroxides, it is nonhygroscopic. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from and release O2 to the atmosphere in spacecraft.
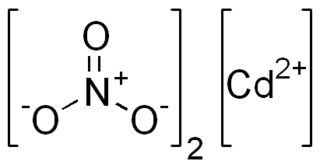
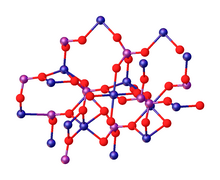
Cadmium nitrate describes any of the related members of a family of inorganic compounds with the general formula Cd(NO3)2·xH2O. The most commonly encountered form being the tetrahydrate.The anhydrous form is volatile, but the others are colourless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic. According to X-ray crystallography, the tetrahydrate features octahedral Cd2+ centers bound to six oxygen ligands.

Nickel nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates have also been reported: Ni(NO3)2.9H2O, Ni(NO3)2.4H2O, and Ni(NO3)2.2H2O.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Cobalt(II) carbonate is the inorganic compound with the formula CoCO3. This reddish paramagnetic solid is an intermediate in the hydrometallurgical purification of cobalt from its ores. It is an inorganic pigment, and a precursor to catalysts. Cobalt(II) carbonate also occurs as the rare red/pink mineral spherocobaltite.
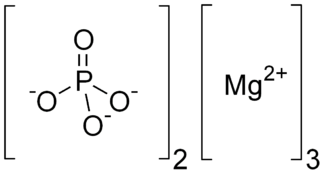
Trimagnesium phosphate describes inorganic compounds with formula Mg3(PO4)2.xH2O. They are magnesium acid salts of phosphoric acid, with varying amounts of water of crystallization: x = 0, 5, 8, 22.

Cobalt(II) sulfate is any of the inorganic compounds with the formula CoSO4(H2O)x. Usually cobalt sulfate refers to the hexa- or heptahydrates CoSO4.6H2O or CoSO4.7H2O, respectively. The heptahydrate is a red solid that is soluble in water and methanol. Since cobalt(II) has an odd number of electrons, its salts are paramagnetic.

Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.

Copper(II) phosphate are inorganic compounds with the formula Cu3(PO4)2. They can be regarded as the cupric salts of phosphoric acid. Anhydrous copper(II) phosphate and a trihydrate are blue solids.

Manganese(II) nitrate refers to the inorganic compounds with formula Mn(NO3)2·(H2O)n. These compounds are nitrate salts containing varying amounts of water. A common derivative is the tetrahydrate, Mn(NO3)2·4H2O, but mono- and hexahydrates are also known as well as the anhydrous compound. Some of these compounds are useful precursors to the oxides of manganese. Typical of a manganese(II) compound, it is a paramagnetic pale pink solid.

Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
Cobalt compounds are chemical compounds formed by cobalt with other elements.