Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram. In minerals, phosphorus generally occurs as phosphate.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Phosphoric acid is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
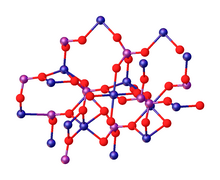
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white solids of nutritious value and are found in many living organisms, e.g., bone mineral and tooth enamel. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals are used in the production of phosphoric acid and fertilizers. Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals).

A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO
4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is H
n+2−2xP
nO
3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and (n+2)/2.

Ammonium phosphate is the inorganic compound with the formula (NH4)3PO4. It is the ammonium salt of orthophosphoric acid. A related "double salt", (NH4)3PO4.(NH4)2HPO4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane and alcohols. The formula for this acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2, which highlights its monoprotic character. Salts derived from this acid are called hypophosphites.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
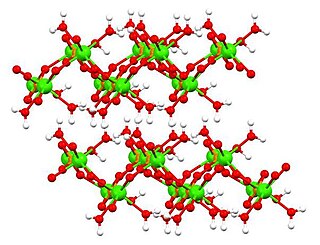
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Dipotassium phosphate (K2HPO4) (also dipotassium hydrogen orthophosphate; potassium phosphate dibasic) is the inorganic compound with the formula K2HPO4.(H2O)x (x = 0, 3, 6). Together with monopotassium phosphate (KH2PO4.(H2O)x), it is often used as a fertilizer, food additive, and buffering agent. It is a white or colorless solid that is soluble in water.

Magnesium phosphate is a general term for salts of magnesium and phosphate appearing in several forms and several hydrates:

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound of sodium with a dihydrogen phosphate (H2PO4−) anion. One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as mono- and dihydrates.

Monomagnesium phosphate is one of the forms of magnesium phosphate. It is a magnesium acid salt of phosphoric acid with the chemical formula Mg(H2PO4)2. Di- and tetrahydrates are known also. It dissolves in water, forming phosphoric acid and depositing a solid precipitate of Mg(HPO4).3H2O, dimagnesium phosphate.
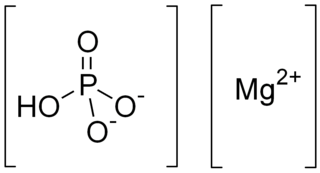
Dimagnesium phosphate is a compound with formula MgHPO4. It is a Mg2+ salt of monohydrogen phosphate. The trihydrate is well known, occurring as a mineral.

Copper(II) phosphate are inorganic compounds with the formula Cu3(PO4)2.n(H2O). They can be regarded as the cupric salt of phosphoric acid. Anhydrous copper(II) phosphate is a blue solid. It is produced by a high-temperature reaction between diammonium phosphate and copper(II) oxide.
Boron phosphate is an inorganic compound with the chemical formula BPO4. The simplest way of producing it is the reaction of phosphoric acid and boric acid. It is a white infusible solid that evaporates above 1450 °C.

Cobalt phosphate is the inorganic compound with the formula Co3(PO4)2. It is a commercial inorganic pigment known as cobalt violet. Thin films of this material are water oxidation catalysts.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.