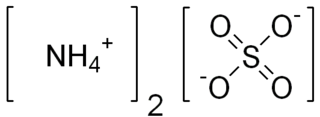Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.
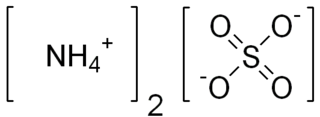
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
Aluminium phosphate is a chemical compound. In nature it occurs as the mineral berlinite. Many synthetic forms of aluminium phosphate are known. They have framework structures similar to zeolites and some are used as catalysts, ion-exchangers or molecular sieves. Commercial aluminium phosphate gel is available.

Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic.

Sodium arsenate is the inorganic compound with the formula Na3AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic. It is usually handled as the dodecahydrate Na3AsO4.12H2O.
Boron phosphate is an inorganic compound with the chemical formula BPO4. The simplest way of producing it is the reaction of phosphoric acid and boric acid. It is a white infusible solid that evaporates above 1450 °C.

Gyrolite, NaCa16(Si23Al)O60(OH)8·14H2O, is a rare silicate mineral (basic sodium calcium silicate hydrate: N-C-S-H, in cement chemist notation) belonging to the class of phyllosilicates. Gyrolite is also often associated with zeolites. It is most commonly found as spherical or radial formations in hydrothermally altered basalt and basaltic tuffs. These formations can be glassy, dull or fibrous in appearance.
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as calcium and rare earth element salts. Minerals in this class were later discovered. Minerals may have sulfite as an essential component, or have it substituted for another anion as in alloriite. The related ions [O3SOSO2]2− and [(O2SO)2SO2]2− may be produced in a reaction between sulfur dioxide and sulfate and exist in the solid form as tetramethyl ammonium salts. They have a significant partial pressure of sulfur dioxide.
The borophosphates are mixed anion compounds containing borate and phosphate anions, which may be joined together by a common oxygen atom. Compounds that contain water or hydroxy groups can also be included in the class of compounds.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.
Curium(III) nitrate is an inorganic compound, a salt of curium and nitric acid with the chemical formula Cm(NO3)3.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Promethium(III) nitrate is an inorganic compound, a salt of promethium and nitric acid with the chemical formula Pm(NO3)3. The compound is radioactive, soluble in water and forms crystalline hydrates.
A phosphate phosphite is a chemical compound or salt that contains phosphate and phosphite anions (PO33- and PO43-). These are mixed anion compounds or mixed valence compounds. Some have third anions.

Neodymium acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). In these compounds, terbium generally exhibits the +3 oxidation state, such as TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form in the 0, +1 and +2 oxidation states.

Promethium(III) bromide is an inorganic compound, with the chemical formula of PmBr3. It is radioactive salt. It is a crystal of the hexagonal crystal system, with the space group of P63/mc (No. 176).